Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Divide the matrix by b, and find the new matrix? If the diagonal elements are represented with x, what is x in terms of a,
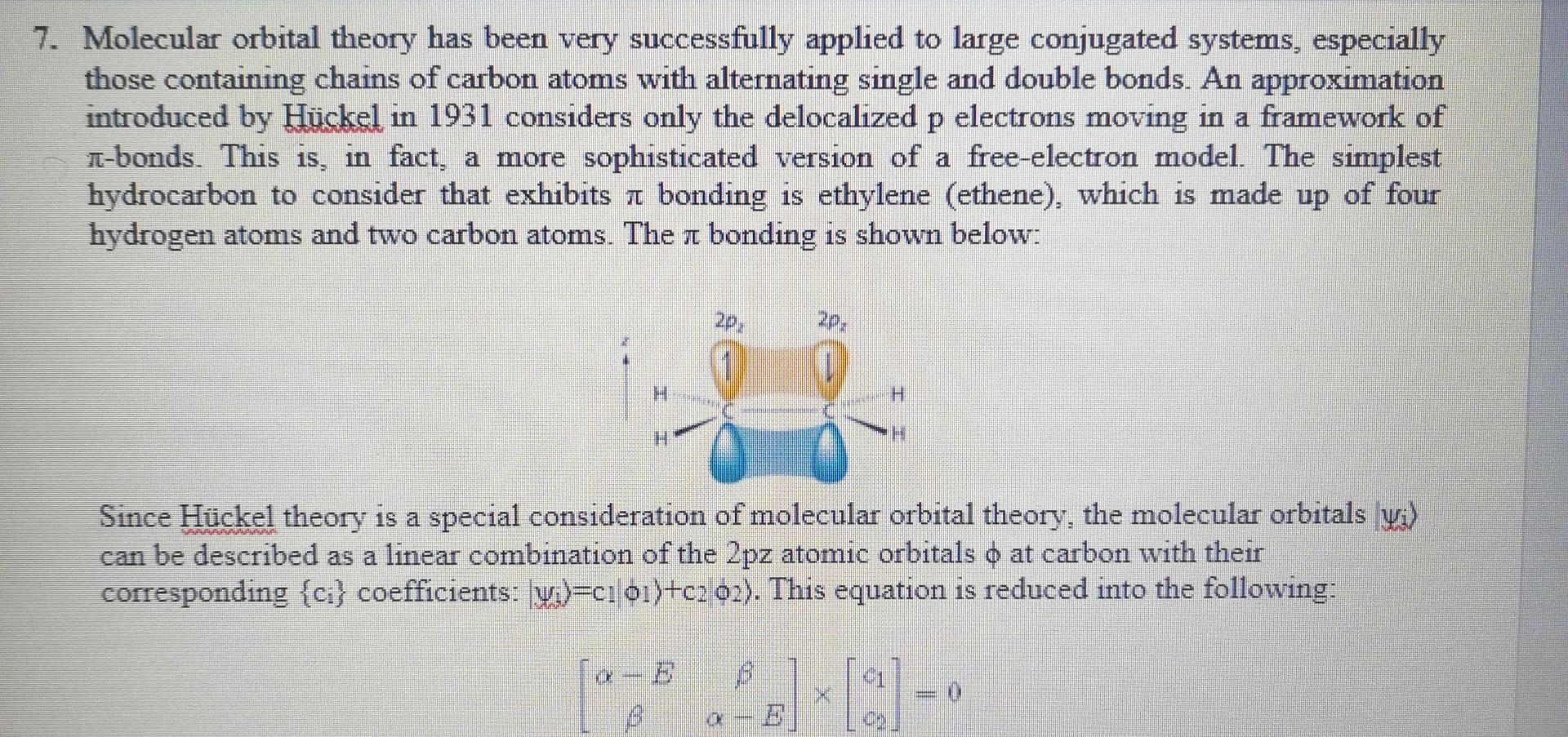
- Divide the matrix by b, and find the new matrix?
- If the diagonal elements are represented with x, what is x in terms of a, b, and E.
- Update the matrix using x on diagonal elements?
- Find the determinant of the matrix and solutions for x?
- Now represent E in terms of a and b by replacing the values of x.
- Now find |1 using the positive value of x and |2 using negative value of x.
- Assuming |1 corresponds to HOMO and 2 corresponds to LUMO, what is the ELUMOEHOMO?
- Normalize each molecular orbital wave function if you can, what is the normalization coefficient?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


