Answered step by step
Verified Expert Solution
Question
1 Approved Answer
DO NOT COPY OTHER CHEGG ANSWERS. I POSTED THIS QUESTION 3 TIMES AND EVERY EXPERT COPIED A WRONG ANSWER. PLEASE ANSWER IT YOURSELF. AND PLEEEEEEASE
DO NOT COPY OTHER CHEGG ANSWERS. I POSTED THIS QUESTION 3 TIMES AND EVERY EXPERT COPIED A WRONG ANSWER. PLEASE ANSWER IT YOURSELF. AND PLEEEEEEASE INCLUDE THE MATLAB PART!!!!!!
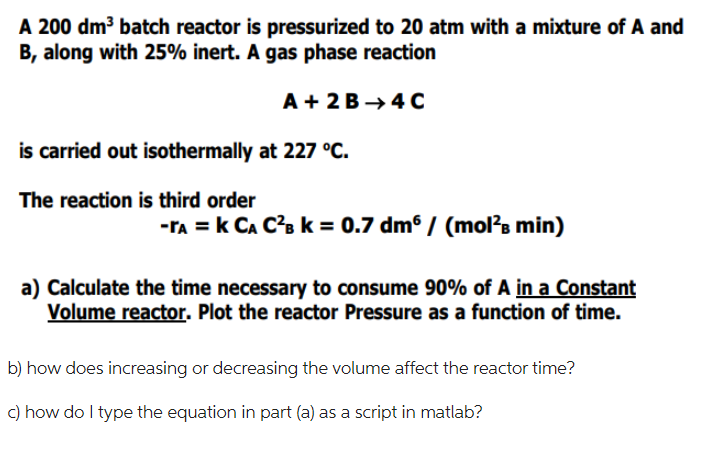
A 200dm3 batch reactor is pressurized to 20atm with a mixture of A and B, along with 25% inert. A gas phase reaction A+2B4C is carried out isothermally at 227C. The reaction is third order rA=kCAC2k=0.7dm6/(mol2min) a) Calculate the time necessary to consume 90% of A in a Constant Volume reactor. Plot the reactor Pressure as a function of time. b) how does increasing or decreasing the volume affect the reactor time? c) how do I type the equation in part (a) as a script in matlabStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


