Answered step by step
Verified Expert Solution
Question
1 Approved Answer
do the calculations required for trial 1 Trial #2 15,8185 15, 2013 061720 Trial #3 15,2013 14, 6899 0,5114 0.2 mold Table 1: Determination of
do the calculations required for trial 1 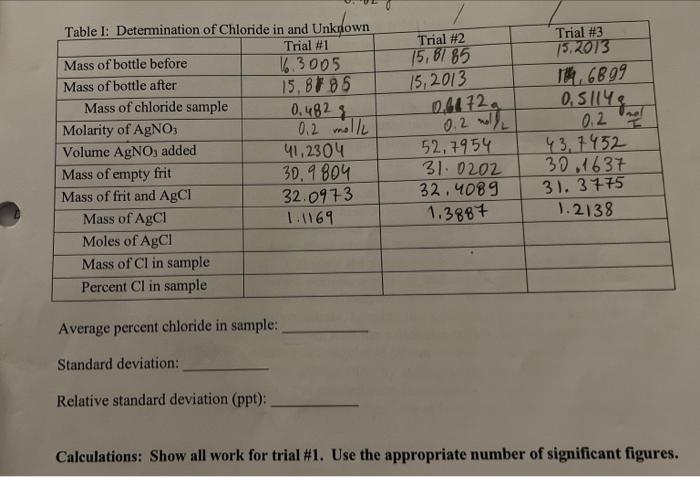
Trial #2 15,8185 15, 2013 061720 Trial #3 15,2013 14, 6899 0,5114 0.2 mold Table 1: Determination of Chloride in and Unknown Trial #1 Mass of bottle before 16.3005 Mass of bottle after 15,87 85 Mass of chloride sample 0.4823 Molarity of AgNO3 0,2 moll Volume AgNO, added 41,2304 Mass of empty frit 30,9804 Mass of frit and AgCl 32.0973 Mass of AgCl 1.1169 Moles of AgCI Mass of Cl in sample Percent Cl in sample 52,7954 31.0202 32, 4089 1.3887 0.2 m 43,7452 30.1637 31. 3775 1.2138 Average percent chloride in sample: Standard deviation: Relative standard deviation (ppt): Calculations: Show all work for trial #1. Use the appropriate number of significant figures. Trial #2 15,8185 15, 2013 061720 Trial #3 15,2013 14, 6899 0,5114 0.2 mold Table 1: Determination of Chloride in and Unknown Trial #1 Mass of bottle before 16.3005 Mass of bottle after 15,87 85 Mass of chloride sample 0.4823 Molarity of AgNO3 0,2 moll Volume AgNO, added 41,2304 Mass of empty frit 30,9804 Mass of frit and AgCl 32.0973 Mass of AgCl 1.1169 Moles of AgCI Mass of Cl in sample Percent Cl in sample 52,7954 31.0202 32, 4089 1.3887 0.2 m 43,7452 30.1637 31. 3775 1.2138 Average percent chloride in sample: Standard deviation: Relative standard deviation (ppt): Calculations: Show all work for trial #1. Use the appropriate number of significant figures 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


