7.54 In Problem 7.20 (Section 7.13) we saw that acid-catalyzed dehydration of 2,2-dimethylcyclohexanol afforded 1,2- dimethylcyclohexene. To explain this product we must write a
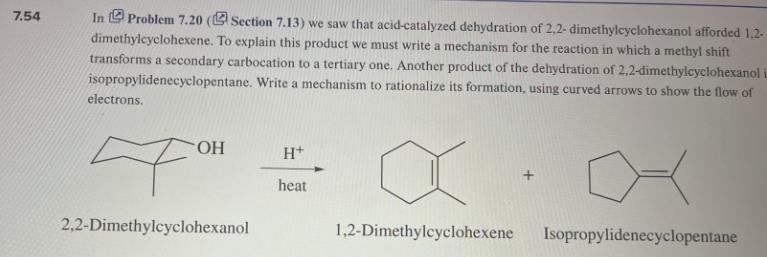
7.54 In Problem 7.20 (Section 7.13) we saw that acid-catalyzed dehydration of 2,2-dimethylcyclohexanol afforded 1,2- dimethylcyclohexene. To explain this product we must write a mechanism for the reaction in which a methyl shift transforms a secondary carbocation to a tertiary one. Another product of the dehydration of 2,2-dimethylcyclohexanol is isopropylidenecyclopentane. Write a mechanism to rationalize its formation, using curved arrows to show the flow of electrons. OH 2,2-Dimethylcyclohexanol H+ heat 1,2-Dimethylcyclohexene Isopropylidenecyclopentane
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To explain the formation of 12dimethylcyclohexene and isopropylidene cyclopentane from 22dimethylcyclohexanol under acidcatalyzed dehydration conditio...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


