Answered step by step
Verified Expert Solution
Question
1 Approved Answer
does anyone know what these answers would be for this lab report? Solubility in Water Complete the table using your observations. Aqueous Solutions Water is
does anyone know what these answers would be for this lab report? 
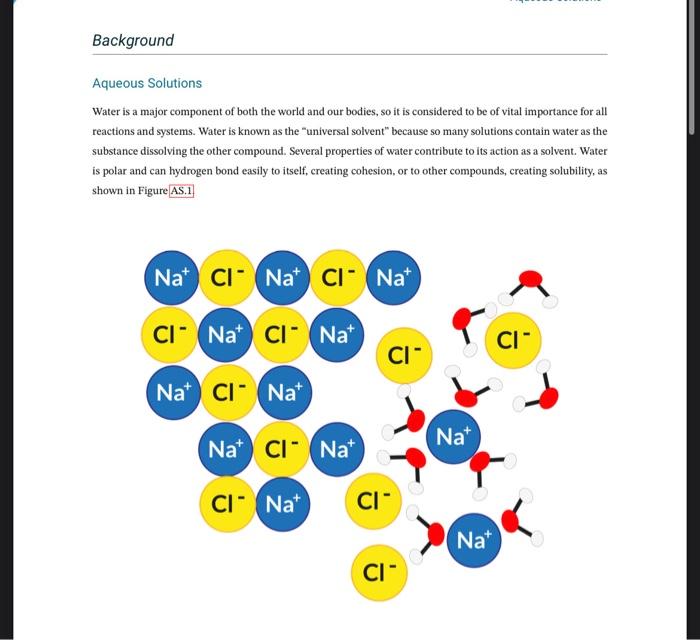



Solubility in Water Complete the table using your observations. Aqueous Solutions Water is a major component of both the world and our bodies, so it is considered to be of vital importance for all reactions and systems. Water is known as the "universal solvent" because so many solutions contain water as the substance dissolving the other compound. Several properties of water contribute to its action as a solvent. Water is polar and can hydrogen bond easily to itself, creating cohesion, or to other compounds, creating solubility, as shown in Figure Figure AS.1: Dissolution of sodium chloride showing attraction of water to ions Solutions are homogeneous mixtures. They tend to be clear and maybe colorless or not, depending on what is dissolved. The major component in a solution is considered the solvent and the minor components are considered to be the solute. An example of a complex solution that we are all familiar with is air. The major component is As.2 Aqueous Solutions nitrogen, which makes it the solvent of the solution. Oxygen and carbon dioxide are minor components and are considered to be solutes. Solubility is the extent to which a solute will dissolve in a given solvent to create a solution. If two components can mix at any ratio to become a solution, they are considered miscible while those that cannot mix at any ratio are said to be insoluble. Some solutes are soluble under only specific conditions, such as temperature or concentration. A solution containing the maximum concentration of a solute at a given temperature is a saturated solution. Solubility depends on the properties of the solvent and the solute as well as the temperature of the solution. A general rule of thumb for the compatibility of solvents and solutes is "like dissolves like," indicating that polar solutes are generally soluble in polar solvents and non-polar solutes are generally soluble in non-polar solvents. When considering temperature, as a general rule, the warmer a solvent is, the better it will dissolve the solute. If a solvent is warm and dissolves as much solute as possible, cooling the solution will either cause solute to crash out or, if no solute crashes out, the solution is supersaturated. Supersaturated solutions can then be caused to crystallize out the solute by the addition of any more solute. In this experiment, solubility of several molecules in water will be tested. Factors such as stirring, heating, and particle size will be evaluated for their effect on solubility. The effect of saturation and supersaturation will be observed as well as the impact of solvent on reaction success. Safety Precautions Safety goggles are required! Ethanol is flammable and toxic. Naphthalene is flammable and a possible carcinogen. Sodium sulfate is a hydroscopic irritant. Glycerol is a hydroseopic irritant. Urea is an irritant. Sodium thiosulfate is a hydroscopic irritant. Citric acid is an irritant. Aqueous Solutions 1. Create a hot water bath in a 250mL beaker on the bot plate by heating water to boiling for later use. Solubility in Water 1. Tube 1: sodium sulfate (Na2SO4) 2. Tube 2: Barium sulfate ( BaSO4) 3. Tube 3: finely ground sucrose (C12H22O11) 4. Tube 4: naphthalene (C20H8) 5. Tube 5: urea (CH4N2O) 6. Tube 6: ethanol (C2H5OH) 7. Tube 7: vegetable oil 8. Tube 8: glycerol (C3HsO3) 1. Add 1.0mL of deionized water ( 20 drops) to each test tube. 2. Add the appropriate solute to each labelled test tube. If the solute is liquid, add 5 drops, if the solute is solid, add an amount the size of the spatula tip. 3. Mix each solution thoroughly. One way to do so is to hold a gloved thumb over the opening and invert the tube several times. 4. Record your observations on the data sheet indicating whether the solute is soluble or not in water. 5. Discard the waste in the appropriate waste container and wash the test tubes with soap and water. Factors Affecting Solubility 1. Tube 1: lump sucrose, room temp, unmixed 2. Tube 2: lump sucrose, room temp, mixed 3. Tube 3: lump sucrose, heated, unmixed 4. Tube 4: fine ground sucrose, room temp, unmixed 5. Tube 5: fine ground sucrose, room temp, mixed 6. Tube 6: fine ground sucrose, heated, unmixed 1. Add 5.0mL of deionized water to each tube. 2. Place tubes 3 and 6 in the hot water bath. 3. Add the lump sucrose to tubes 13 and fine ground sucrose to tubes 46. Mix ONLY tubes 2 and 5 , do not mix the other tubes. 4. Start timing the amount of time it takes to dissolve the sucrose. 5. Record the amount of time it takes to dissolve the sucrose in each tube. If a tube takes longer than 6 minutes to dissolve, write >6 minutes on your observations, 6. Dispose of the sucrose solutions in the appropriate waste container and wash the test tubes with soap and water. Solubility in Water Complete the table using your observations. Aqueous Solutions Water is a major component of both the world and our bodies, so it is considered to be of vital importance for all reactions and systems. Water is known as the "universal solvent" because so many solutions contain water as the substance dissolving the other compound. Several properties of water contribute to its action as a solvent. Water is polar and can hydrogen bond easily to itself, creating cohesion, or to other compounds, creating solubility, as shown in Figure Figure AS.1: Dissolution of sodium chloride showing attraction of water to ions Solutions are homogeneous mixtures. They tend to be clear and maybe colorless or not, depending on what is dissolved. The major component in a solution is considered the solvent and the minor components are considered to be the solute. An example of a complex solution that we are all familiar with is air. The major component is As.2 Aqueous Solutions nitrogen, which makes it the solvent of the solution. Oxygen and carbon dioxide are minor components and are considered to be solutes. Solubility is the extent to which a solute will dissolve in a given solvent to create a solution. If two components can mix at any ratio to become a solution, they are considered miscible while those that cannot mix at any ratio are said to be insoluble. Some solutes are soluble under only specific conditions, such as temperature or concentration. A solution containing the maximum concentration of a solute at a given temperature is a saturated solution. Solubility depends on the properties of the solvent and the solute as well as the temperature of the solution. A general rule of thumb for the compatibility of solvents and solutes is "like dissolves like," indicating that polar solutes are generally soluble in polar solvents and non-polar solutes are generally soluble in non-polar solvents. When considering temperature, as a general rule, the warmer a solvent is, the better it will dissolve the solute. If a solvent is warm and dissolves as much solute as possible, cooling the solution will either cause solute to crash out or, if no solute crashes out, the solution is supersaturated. Supersaturated solutions can then be caused to crystallize out the solute by the addition of any more solute. In this experiment, solubility of several molecules in water will be tested. Factors such as stirring, heating, and particle size will be evaluated for their effect on solubility. The effect of saturation and supersaturation will be observed as well as the impact of solvent on reaction success. Safety Precautions Safety goggles are required! Ethanol is flammable and toxic. Naphthalene is flammable and a possible carcinogen. Sodium sulfate is a hydroscopic irritant. Glycerol is a hydroseopic irritant. Urea is an irritant. Sodium thiosulfate is a hydroscopic irritant. Citric acid is an irritant. Aqueous Solutions 1. Create a hot water bath in a 250mL beaker on the bot plate by heating water to boiling for later use. Solubility in Water 1. Tube 1: sodium sulfate (Na2SO4) 2. Tube 2: Barium sulfate ( BaSO4) 3. Tube 3: finely ground sucrose (C12H22O11) 4. Tube 4: naphthalene (C20H8) 5. Tube 5: urea (CH4N2O) 6. Tube 6: ethanol (C2H5OH) 7. Tube 7: vegetable oil 8. Tube 8: glycerol (C3HsO3) 1. Add 1.0mL of deionized water ( 20 drops) to each test tube. 2. Add the appropriate solute to each labelled test tube. If the solute is liquid, add 5 drops, if the solute is solid, add an amount the size of the spatula tip. 3. Mix each solution thoroughly. One way to do so is to hold a gloved thumb over the opening and invert the tube several times. 4. Record your observations on the data sheet indicating whether the solute is soluble or not in water. 5. Discard the waste in the appropriate waste container and wash the test tubes with soap and water. Factors Affecting Solubility 1. Tube 1: lump sucrose, room temp, unmixed 2. Tube 2: lump sucrose, room temp, mixed 3. Tube 3: lump sucrose, heated, unmixed 4. Tube 4: fine ground sucrose, room temp, unmixed 5. Tube 5: fine ground sucrose, room temp, mixed 6. Tube 6: fine ground sucrose, heated, unmixed 1. Add 5.0mL of deionized water to each tube. 2. Place tubes 3 and 6 in the hot water bath. 3. Add the lump sucrose to tubes 13 and fine ground sucrose to tubes 46. Mix ONLY tubes 2 and 5 , do not mix the other tubes. 4. Start timing the amount of time it takes to dissolve the sucrose. 5. Record the amount of time it takes to dissolve the sucrose in each tube. If a tube takes longer than 6 minutes to dissolve, write >6 minutes on your observations, 6. Dispose of the sucrose solutions in the appropriate waste container and wash the test tubes with soap and water 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


