Answered step by step
Verified Expert Solution
Question
1 Approved Answer
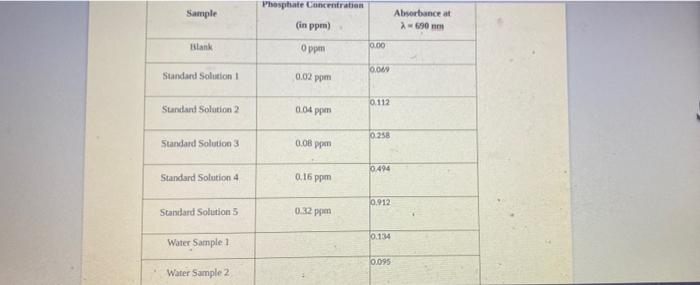
does this help? the formula is (c1)(v1)=(c2)(v2)trying to find/ fill in the answers in the empty boxes Phosphate Concentration Sample Absorbance at 690 mm (in
does this help? 

the formula is (c1)(v1)=(c2)(v2)trying to find/ fill in the answers in the empty boxes
Phosphate Concentration Sample Absorbance at 690 mm (in ppm) Blank 0.00 0.00 Standard Solution 0.02 pm 0.112 Sundand Solution 2 004 pm 0.258 Standard Solution 3 0.08 pon 0.494 Standard Solution 4 0.16 ppm 0.912 Standard Solution 3 0.32 ppm 0.134 Water Sample 1 0.095 Water Sample 2 1) Construct a phosphate standard curve in Excel by plotting concentration (in ppn) on your -axis and Absorbance (unidess) on your y-axis for your known solutions. Label the axes on the graph and provide the curve with a title. Use a linear trendline to generate a best fit line to your data. Label the graph with the equation and the value. Insert your labeled graph in the space below. 2) Using the equation from your graph, determine the phosphate concentration (in ppm) in each of your natural water samples Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


