Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. Draw the and o* molecular orbitals for discrete and localized bonds formed between hybridized carbons and hetero- atoms (a heteroatom is any atom
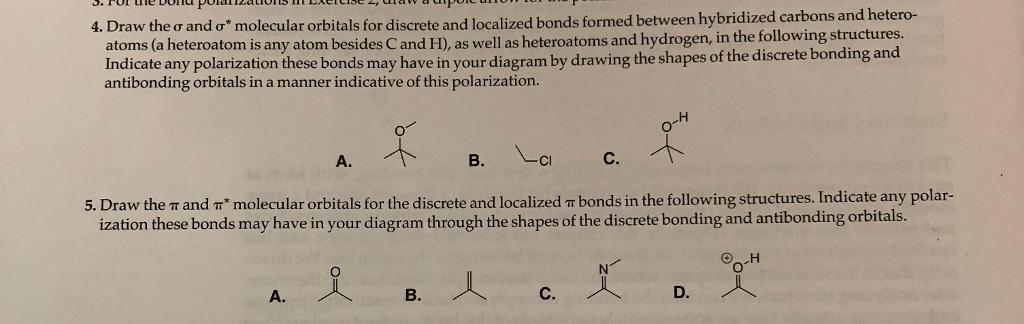
4. Draw the and o* molecular orbitals for discrete and localized bonds formed between hybridized carbons and hetero- atoms (a heteroatom is any atom besides C and H), as well as heteroatoms and hydrogen, in the following structures. Indicate any polarization these bonds may have in your diagram by drawing the shapes of the discrete bonding and antibonding orbitals in a manner indicative of this polarization. LCI 5. Draw the T and T* molecular orbitals for the discrete and localized bonds in the following structures. Indicate any polar- ization these bonds may have in your diagram through the shapes of the discrete bonding and antibonding orbitals. -H X A. A. B. B. C. D.
Step by Step Solution
★★★★★
3.55 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
molecular orbitals are formed via the headon overlap of two orbitals see below omolecular orbitals b...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


