Question
Draw the condensed structural formula for the structure given on the CHEM 1152 worksheet for 1b and answer the following questions . a) Give the
Draw the condensed structural formula for the structure given on the CHEM 1152 worksheet for 1b and answer the following questions .
a) Give the molecular formula (not the structure ) for this compound (C_{X}*H_{y}) (to write the molecular formula, click the arrow next to the Bold, italic, underline icons to find the subscript button, hit again to get out of that mode) You can use this function for writing our condensed molecular structures also. 1 pt
b) Using the molecular formula, write out the balanced combustion reaction (There is an exercise in iCollege that explains how to balance combustion reactions , refer to this sheet ). 3 pts
c ) State the polarity (polar or nonpolar) of this compound and it's solubility in water (water is a polar compound). Explain. 4 pts
d) Give the IUPAC name for this compound. 2 pts
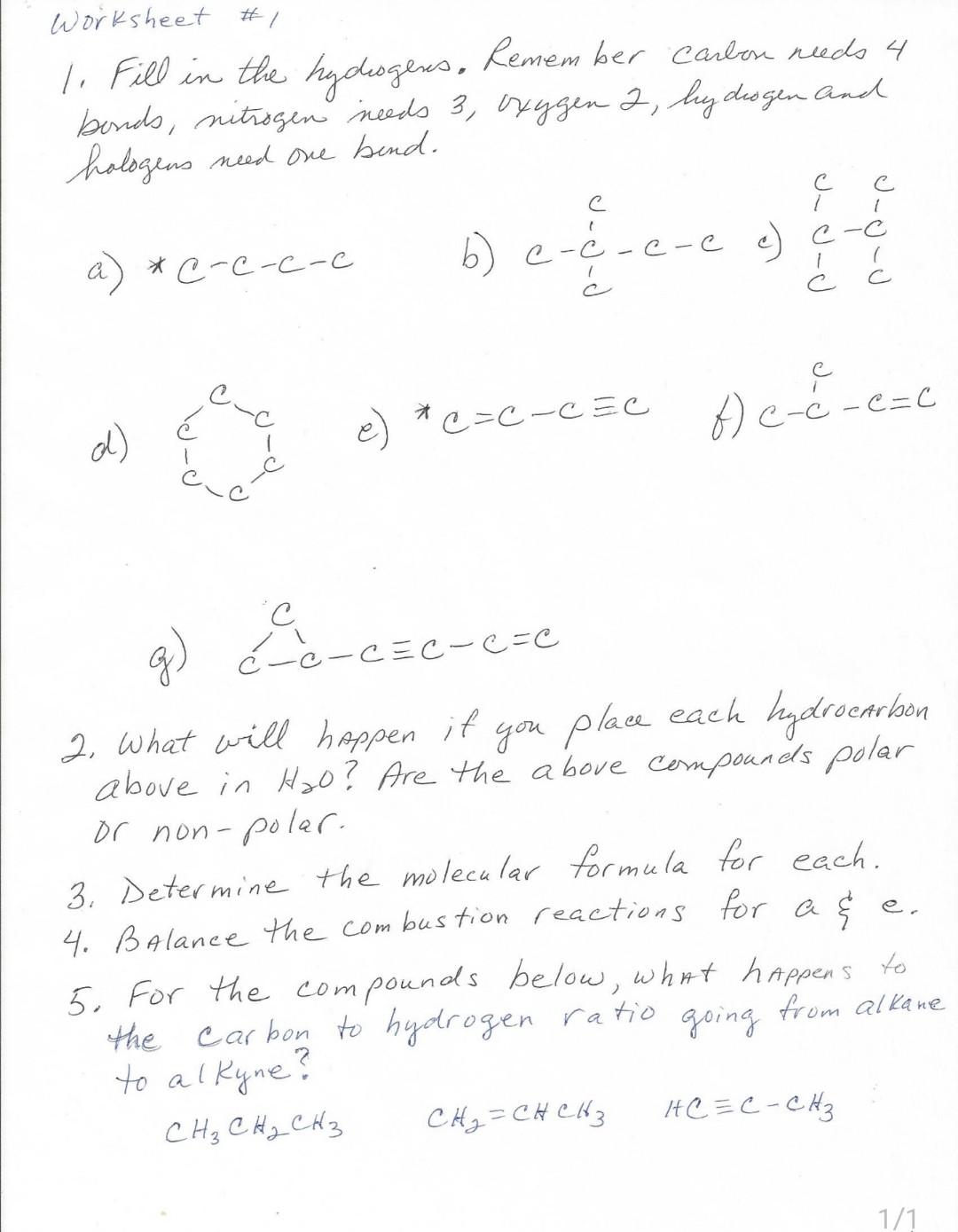
Worksheet #1 1. Fill in the hydoogers, Remember Carbon redo 4 bonds, nitrogen needs 3, oxygen 2, hydrogen and halogens need one bend. a) *c-c-c-c 6) e-e-e-ee) e-6 C 1 ( 1 f) e-c-e=c o e) *-C & c =C-CEC d) g) Frencserece --= 2. What will happen if you place each hydrocarbon above in HO? Are the above compounds polar or non-polar- 3. Determine the molecular formula for each. 4. Balance the combustion reactions for a $ 5. For the compounds below, what happens to the carbon to hydrogen ratio going from alkane to alkyne? CH CH CH3 CH = CH CH 3 AC = C-CH e. 1/1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


