Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Draw the Lewis structure of AsO4^3? showing alllone pairs. Identify the molecular geometry of AsO4^3?.What is the hybridization of thecentral As atom? What are the
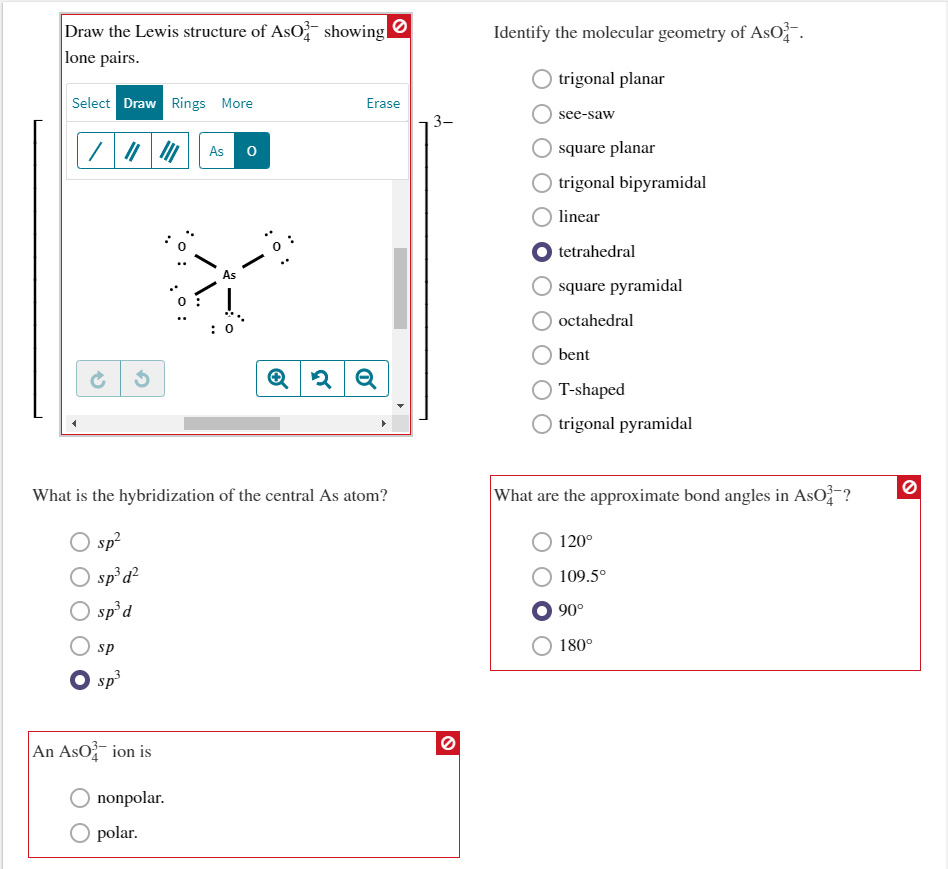
Draw the Lewis structure of AsO4^3? showing alllone pairs. Identify the molecular geometry of AsO4^3?.What is the hybridization of thecentral As atom? What are the approximate bond angles in AsO4^3??
An AsO4^3? ion is polar or non polar((On the homework feedback, it said it is a valid structure but notthe most stable. But I can't figure out why.))
Draw the Lewis structure of AsO showing lone pairs. Select Draw Rings More sp Osp An AsO ion is 0: O nonpolar. polar. As 0 As : 0 What is the hybridization of the central As atom? sp sp d sp d Erase Q2 Q O Identify the molecular geometry of AsO. O trigonal planar see-saw square planar trigonal bipyramidal linear tetrahedral square pyramidal octahedral bent T-shaped O trigonal pyramidal What are the approximate bond angles in AsO? 120 109.5 90 180 O
Step by Step Solution
★★★★★
3.44 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
7 3 lewis structure of Asoy 00 0C60 As bond angle...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


