Question
Draw the Lewis structure of SF4 showing all lone pairs. What is the hybridization of the central atom? sp spd spd An SF4 molecule
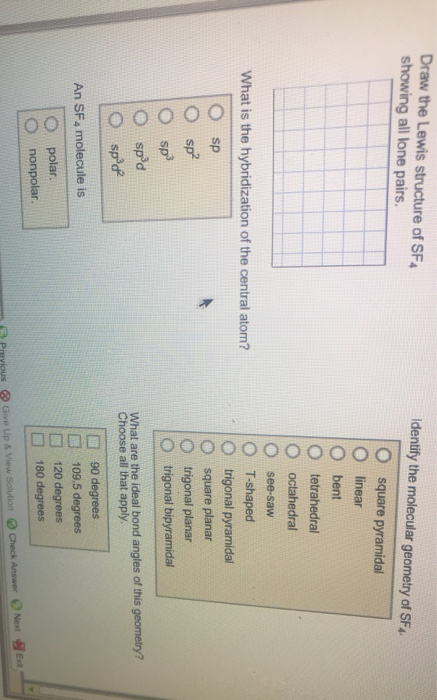
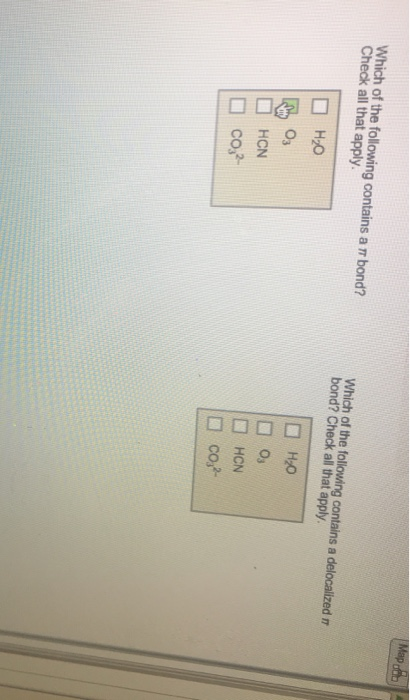
Draw the Lewis structure of SF4 showing all lone pairs. What is the hybridization of the central atom? sp spd spd An SF4 molecule is polar. nonpolar. Identify the molecular geometry of SF4. square pyramidal linear bent tetrahedral octahedral see-saw T-shaped trigonal pyramidal square planar trigonal planar trigonal bipyramidal What are the ideal bond angles of this geometry? Choose all that apply. 90 degrees 109.5 degrees 120 degrees 180 degrees yious Give Up & View Solulion Check Answer Next Exit Which of the following contains a r bond? Check all that apply. HO 03 HCN co- Which of the following contains a delocalized n bond? Check all that apply. HO 0 HCN co- Map 000
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Thomas Engel, Philip Reid
3rd edition
805338423, 080533842X, 978-0321812001
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



