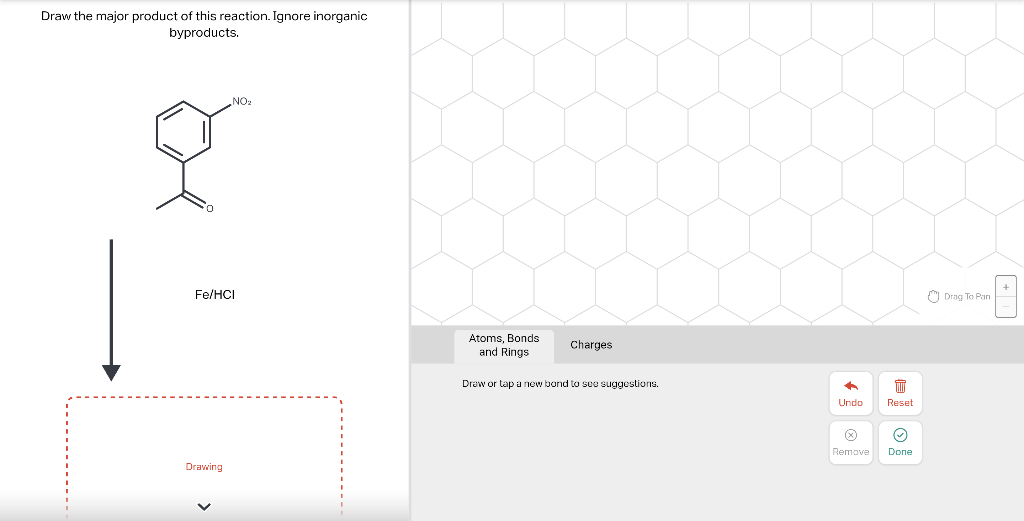
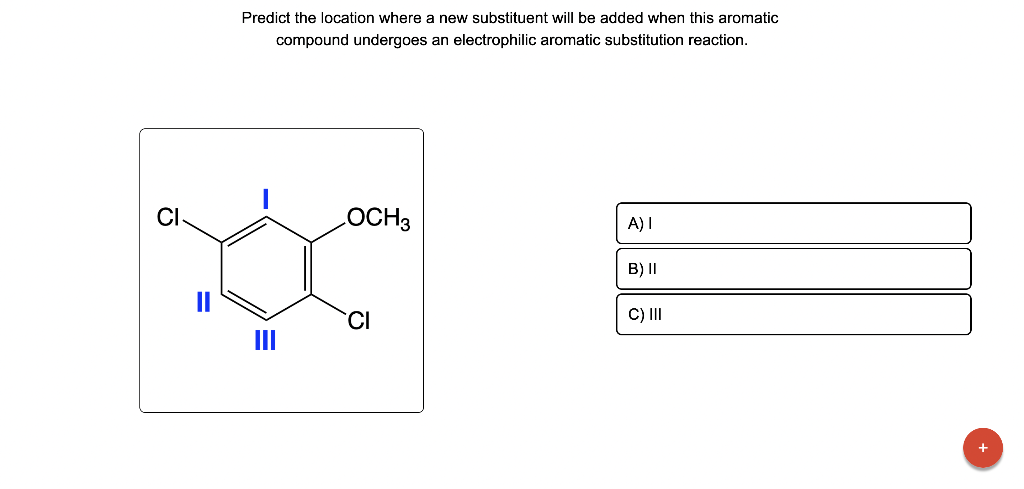
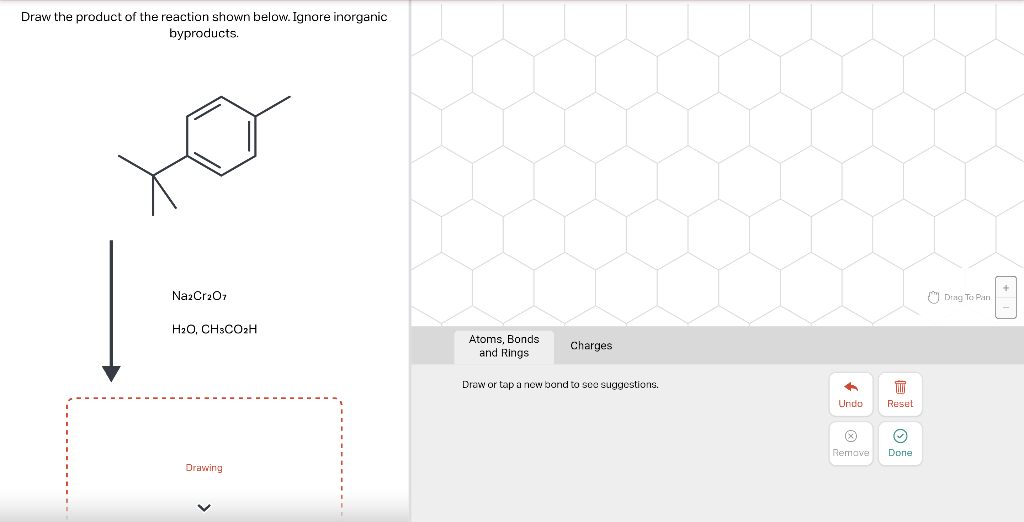
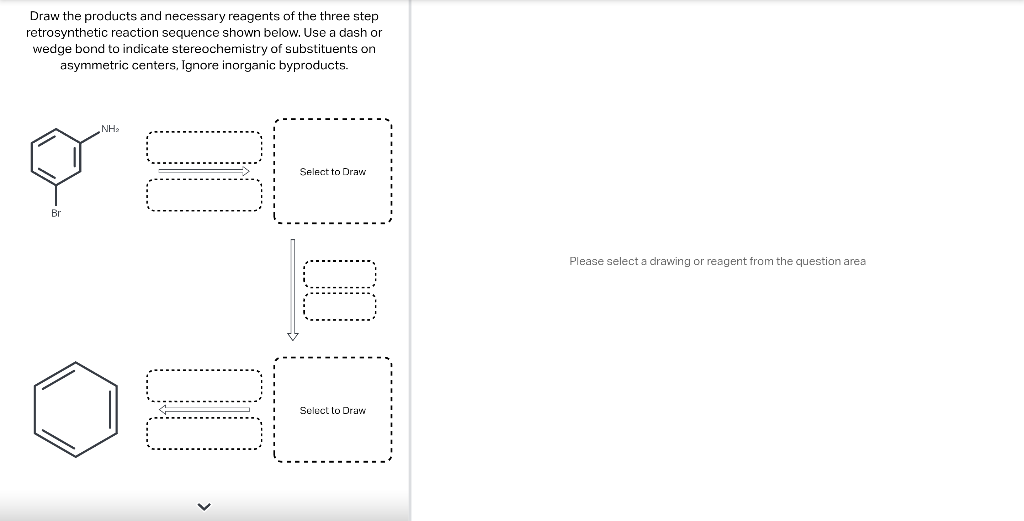
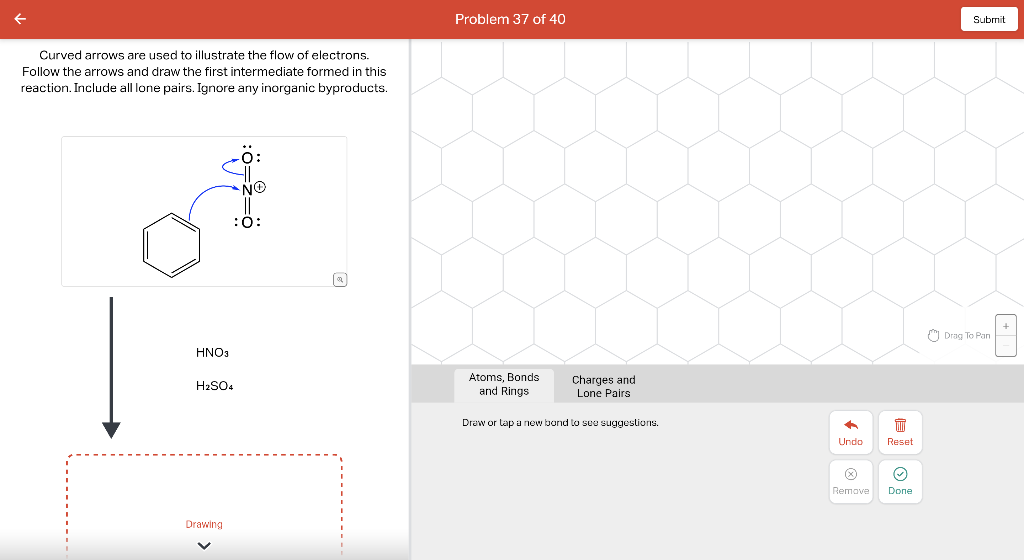
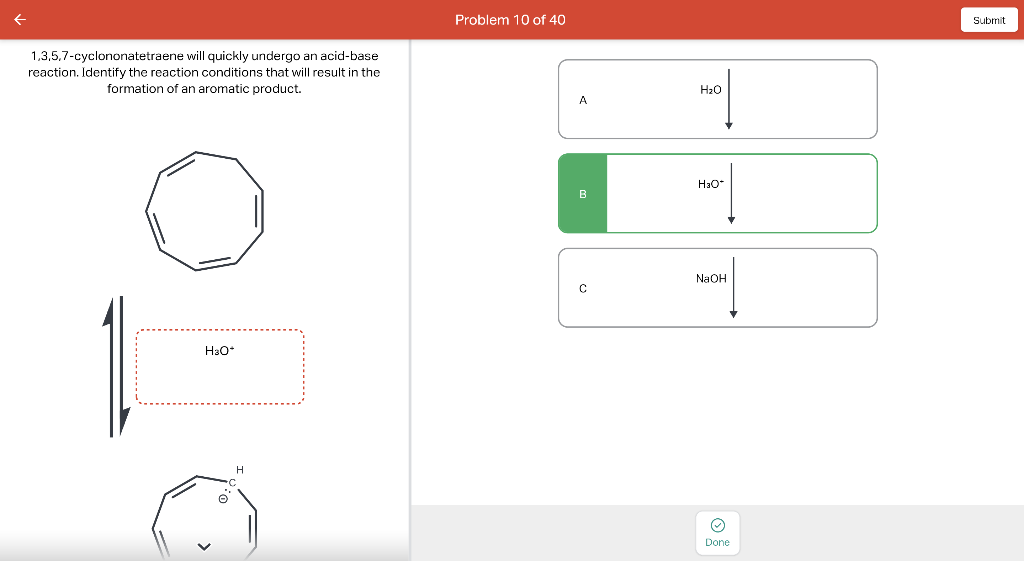
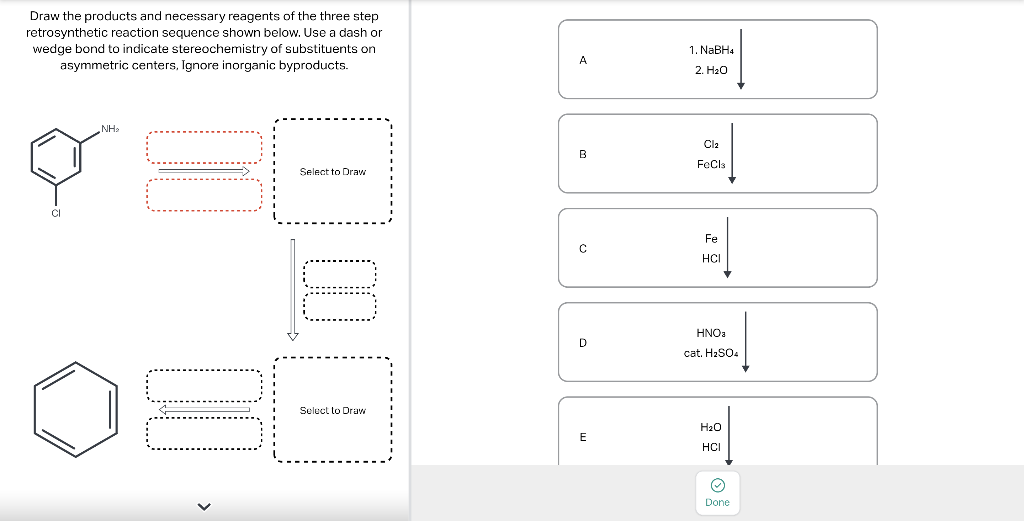
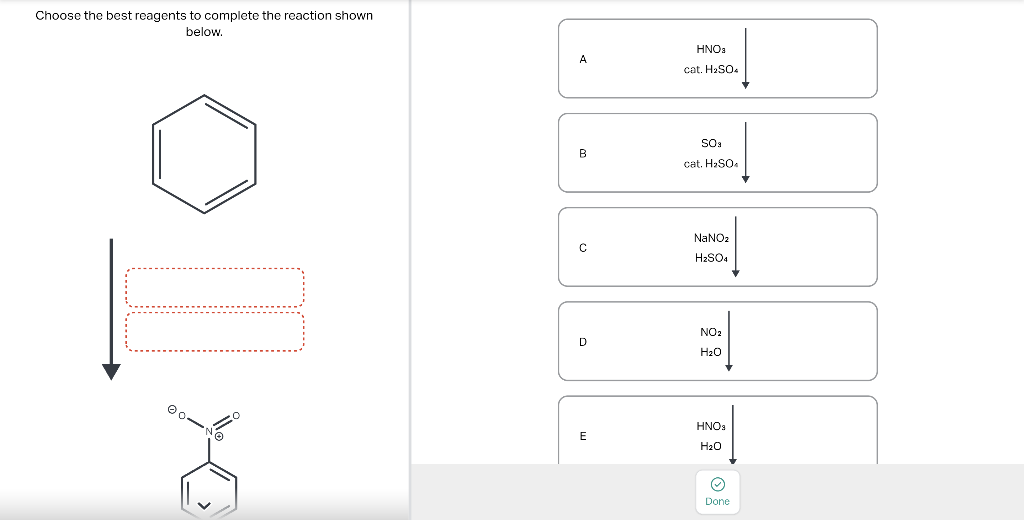
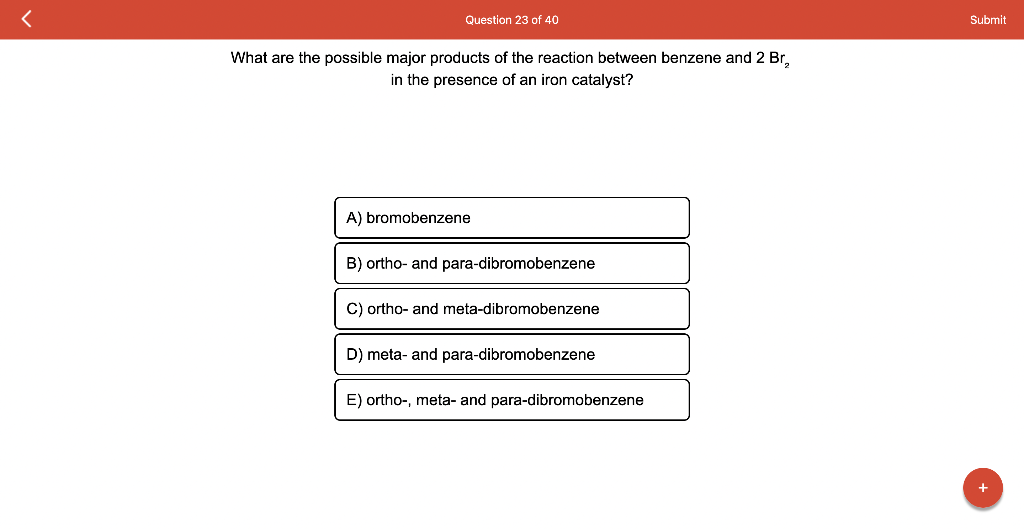
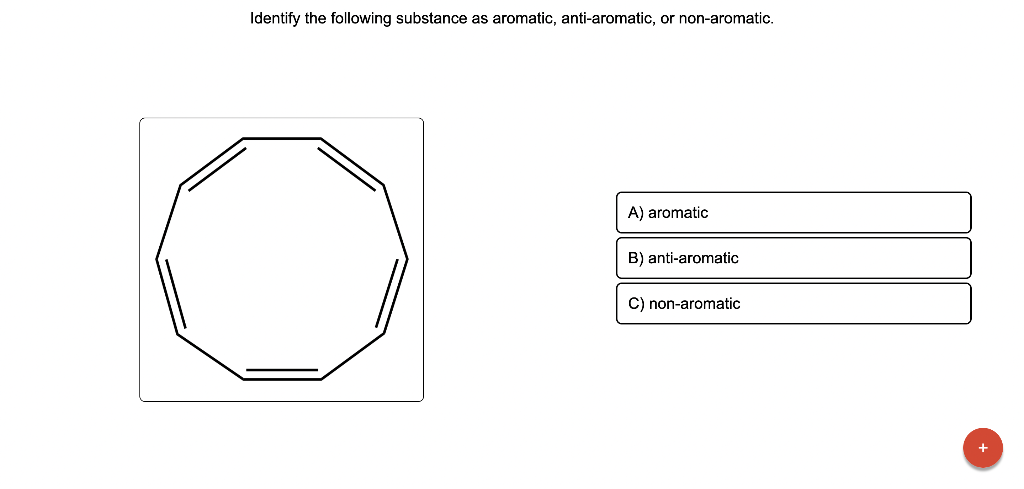

Draw the major product of this reaction. Ignore inorganic Draw or tap a new bond to see suggestions. Predict the location where a new substituent will be added when this aromatic compound undergoes an electrophilic aromatic substitution reaction. Draw the product of the reaction shown below. Ignore inorganic byproducts. Na2Cr2O7 H2O,CH3CO2H Draw or tap a new bond to see suggestions. Draw the products and necessary reagents of the three step retrosynthetic reaction sequence shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproducts. Please select a drawing or reagent from the question area Problem 37 of 40 Curved arrows are used to illustrate the flow of electrons. Follow the arrows and draw the first intermediate formed in this reaction. Include all lone pairs. Ignore any inorganic byproducts. Draw or tap a new bond to see suggestions. 1,3,5,7-cyclononatetraene will quickly undergo an acid-base reaction. Identify the reaction conditions that will result in the formation of an aromatic product. Draw the products and necessary reagents of the three step retrosynthetic reaction sequence shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproducts. Choose the best reagents to complete the reaction shown below. What are the possible major products of the reaction between benzene and 2Br2 in the presence of an iron catalyst? Identify the following substance as aromatic, anti-aromatic, or non-aromatic. Choose the best explanation of why the methoxy group is an o,p-director in electrophilic aromatic substitution reactions. A) The methoxy group donates electron density to the ring by resonance and stabilizes the ortho and para position in the arenium cation intermediate. B) The methoxy group donates electron density to the ring by induction and stabilizes the ortho and para position in the arenium cation intermediate. C) The methoxy group donates electron density to the ring by resonance and destabilizes the ortho and para position in the arenium cation intermediate. D) The methoxy group donates electron density to the ring by induction and destabilizes the ortho and para position in the arenium cation intermediate

















