Drop Method


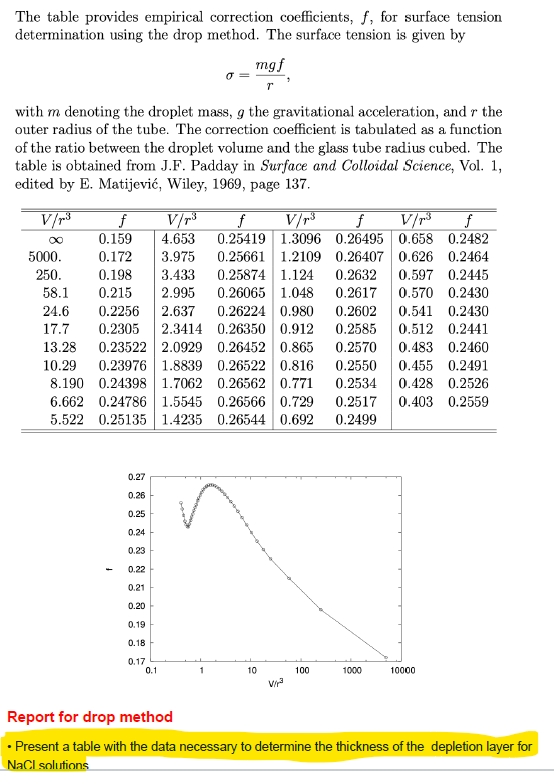

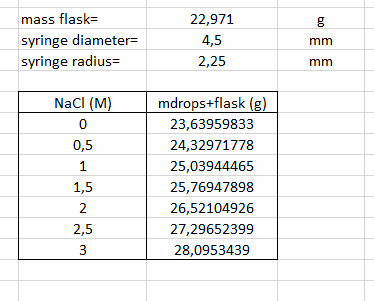
Motorized shaft pushing downward Syringe plunger I. Drop formation point Syringe to be mounted Mounted syringe Mounted syringe (close up) A glass tube is mounted vertically such that the bottom part is open, while the upper part is attached to a motorized syringe. The speed of the motorized syringe is set such that liquid droplets will fall out from the open end of the glass tube at roughly one minute intervals. The drops will be collected and the surface tension will be determined from the weight or volume of the drops. Ideally mg =pVg = 2nro, (14) with m denoting the weight of the solution, V its volume, and p its density. The outer radius of the glass tube is given by r and the gravitational acceler- ation by g. Equation (14) holds assuming a semispherical shape at the time of 'dispatch' and that whole of the drop actually does fall. In practice, this does not hold and one has to introduce an empirical correction coefficient, f. The correction coefficient (tabulated on page 1) is found to depend on the ratio between the volume of the drop and the tube's radius cubed, V/r3. The volume will be determined using the density of the NaCl solution as tabulated on page 10. Using an interpolated value of f (f includes the factor 1/2), the surface tension can be determined as mgf 0 = (15) r Solutions: Dilute the stock solution to obtain seven different salt concen- trations: 0.0, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mole dm-3. Experimental procedure: 1. Remove the syringe and rinse the equipment with distilled water several times. 2. Fill the syringe with distilled water - air bubbles in the upper part of the syringe will not disturb the measurements - and fix the syringe in working position. 3. Weigh a laboratory flask (it does not need to be particularly clean) and place it under the syringe. Make sure that the bottom (i.e., open) end of the glass tube goes into the laboratory flask. With this procedure, the drops are protected against dust and, for example, coughing, which may cause the drops to fall too early. 4. Collect, e.g., ten drops of the solution. Check off each drop on a piece of paper in front of you! Weigh the flask again and determined the weight of a drop. 5. Redo the measurement using the 0.5 M NaCl solution. Always rinse the syringe with the new solution prior to filling it for the measurement. If you are even slightly unsure about the number of drops - redo the measurement! 6. Continue in order of increasing salt concentration. 7. Rinse and fill the syringe with distilled water once you've finished your measurements. Data analysis: Analyze the data using equation (13) and estimate the thickness of the depletion region. Correction factor for the drop method The table provides empirical correction coefficients, f, for surface tension determination using the drop method. The surface tension is given by mgf with m denoting the droplet mass, g the gravitational acceleration, and r the outer radius of the tube. The correction coefficient is tabulated as a function of the ratio between the droplet volume and the glass tube radius cubed. The table is obtained from J.F. Padday in Surface and Colloidal Science, Vol. 1, edited by E. Matijevi, Wiley, 1969, page 137. V/p3 f V/r3 of V/p3 f V/13 f 0.159 4.653 0.25419 1.3096 0.26495 0.658 0.2482 5000. 0.172 3.975 0.25661 1.2109 0.26407 0.626 0.2464 250. 0.198 3.433 0.25874 1.124 0.2632 0.597 0.2445 58.1 0.215 2.995 0.26065 1.048 0.2617 0.570 0.2430 24.6 0.2256 2.637 0.26224 0.980 0.2602 0.541 0.2430 17.7 0.2305 2.3414 0.26350 0.912 0.2585 0.512 0.2441 13.28 0.23522 2.0929 0.26452 0.865 0.2570 0.483 0.2460 10.29 0.23976 1.8839 0.26522 0.816 0.2550 0.455 0.2491 8.190 0.24398 1.7062 0.26562 0.771 0.2534 0.428 0.2526 6.662 0.24786 1.5545 0.26566 0.729 0.2517 0.403 0.2559 5.522 0.25135 1.4235 0.26544 0.692 0.2499 0.27 0.26 0.25 0.24 0.23 0.22 0.21 0.20 0.19 0.18 0.17 0.1 10 100 1000 10000 wa Report for drop method Present a table with the data necessary to determine the thickness of the depletion layer for NaCl solutions - Determine the thickness of the depletion layer. bo mass flask= syringe diameter syringe radius= 22,971 4,5 2,25 mm mm NaCl (M) 0 0,5 1 1,5 2 2,5 3 mdrops+flask (g) 23,63959833 24,32971778 25,03944465 25,76947898 26,52104926 27,29652399 28,0953439











