Answered step by step
Verified Expert Solution
Question
1 Approved Answer
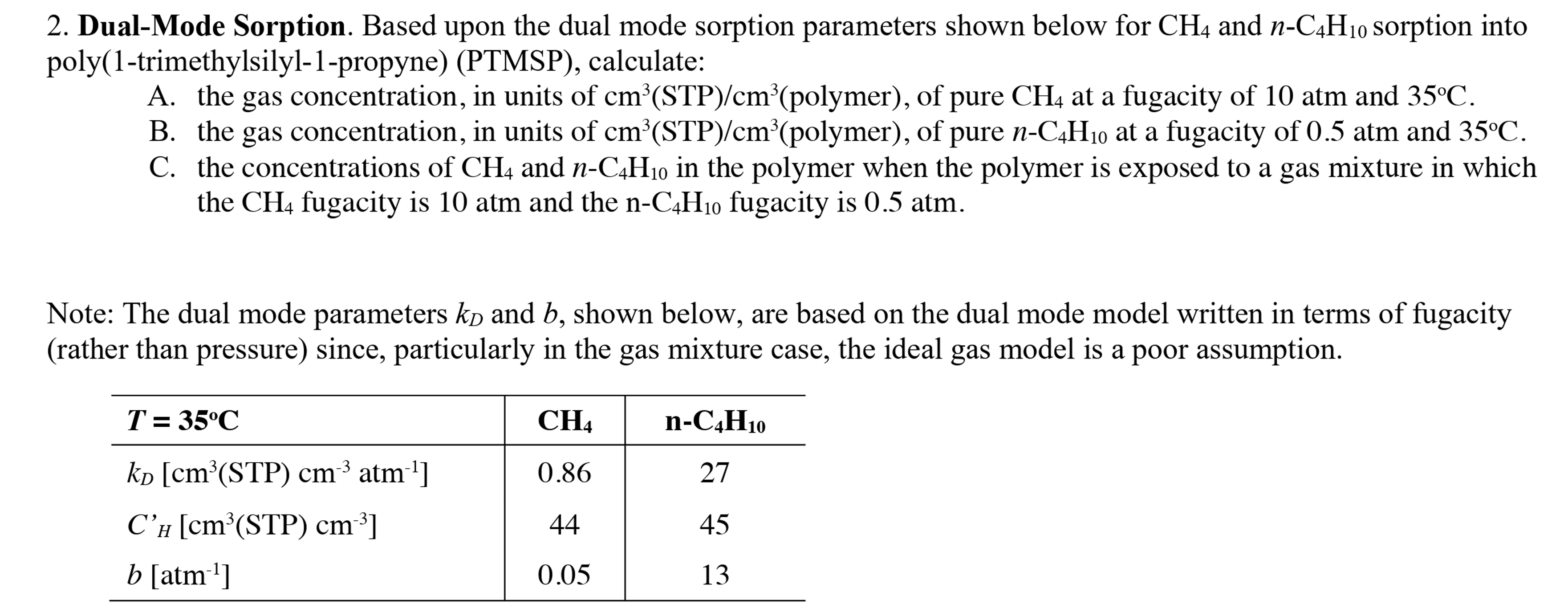
Dual - Mode Sorption. Based upon the dual mode sorption parameters shown below for CH 4 and n - C 4 H 1 0 sorption
DualMode Sorption. Based upon the dual mode sorption parameters shown below for CH and nCH sorption into
polytrimethylsilylpropynePTMSP calculate:
A the gas concentration, in units of cmSTPcmpolymer of pure CH at a fugacity of atm and oC
B the gas concentration, in units of cmSTPcmpolymer of pure nCH at a fugacity of atm and oC
C the concentrations of CH and nCH in the polymer when the polymer is exposed to a gas mixture in which
the CH fugacity is atm and the nCH fugacity is atm.
Note: The dual mode parameters kD and b shown below, are based on the dual mode model written in terms of fugacity DualMode Sorption. Based upon the dual mode sorption parameters shown below for and sorption into
polytrimethylsilylpropynePTMSP calculate:
A the gas concentration, in units of polymer of pure at a fugacity of atm and
B the gas concentration, in units of polymer of pure at a fugacity of atm and
C the concentrations of and in the polymer when the polymer is exposed to a gas mixture in which
the fugacity is atm and the fugacity is atm.
Note: The dual mode parameters and shown below, are based on the dual mode model written in terms of fugacity
rather than pressure since, particularly in the gas mixture case, the ideal gas model is a poor assumption,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


