Answered step by step
Verified Expert Solution
Question
1 Approved Answer
DUE IN LESS THN HOUR! URGENT HELP PLEASE!!!! Be sure to answer all parts. (a) Write a balanced equation for the reaction depicted below. Do
DUE IN LESS THN HOUR! URGENT HELP PLEASE!!!! 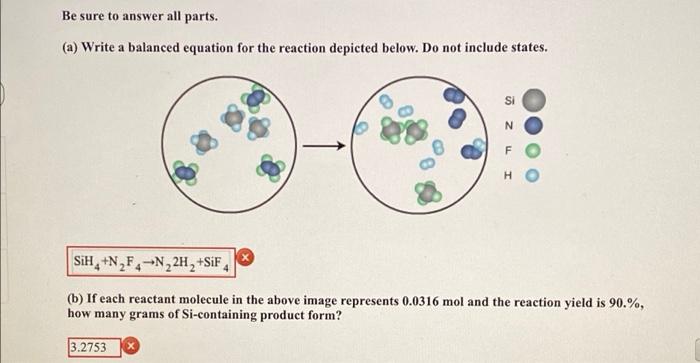



Be sure to answer all parts. (a) Write a balanced equation for the reaction depicted below. Do not include states. a N F H SiH, +N2F4 --N22H, +SiF4 (b) If each reactant molecule in the above image represents 0.0316 mol and the reaction yield is 90.%, how many grams of Si-containing product form? 3.2753 Enter your answer in the provided box. What is the percent yield of a reaction in which 200. g of phosphorus trichloride reacts with excess water to form 85.0 g of HCl and aqueous phosphorous acid (H3PO3)? % yield Be sure to answer all parts. Calcium nitrate and ammonium fluoride react to form calcium fluoride, dinitrogen monoxide, and water vapor. What mass of each substance is present after 17.56 g of calcium nitrate and 18.30 g of ammonium fluoride react completely? g calcium nitrate g ammonium fluoride g calcium fluoride g dinitrogen monoxide g water Be sure to answer all parts. Calculate the maximum numbers of moles and grams of H2S that can form when 161.6 g of aluminum sulfide reacts with 143.0 g of water: Al,S3 + H20 - Al(OH)3 + H2S - (unbalanced] 66.99 h1H 1.97 Is What mass of the excess reactant remains? 72.3 x xcess reactant 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


