Answered step by step
Verified Expert Solution
Question
1 Approved Answer
During the growth of cells in the bioreactor, some heat is also lost generally, the heat loss rate is directly proportional to the cell
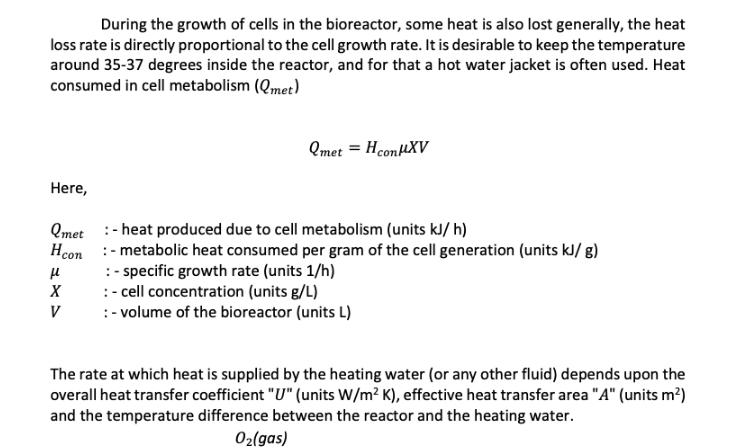
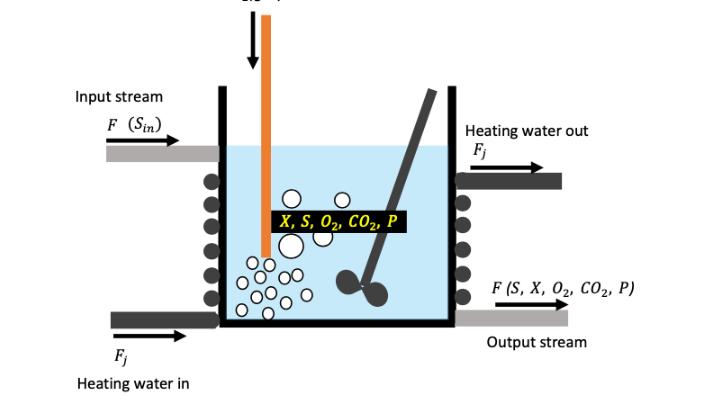
During the growth of cells in the bioreactor, some heat is also lost generally, the heat loss rate is directly proportional to the cell growth rate. It is desirable to keep the temperature around 35-37 degrees inside the reactor, and for that a hot water jacket is often used. Heat consumed in cell metabolism (Qmet) Qmet = H conMXV Here, Qmet - heat produced due to cell metabolism (units kJ/h) Hcon-metabolic heat consumed per gram of the cell generation (units kJ/g) :-specific growth rate (units 1/h) : - cell concentration (units g/L) :- volume of the bioreactor (units L) X V The rate at which heat is supplied by the heating water (or any other fluid) depends upon the overall heat transfer coefficient "U" (units W/m K), effective heat transfer area "A" (units m) and the temperature difference between the reactor and the heating water. O(gas) Input stream F (Sin) Fj Heating water in X, S, O, CO, P Heating water out Fj F (S, X, O, CO, P) Output stream
Step by Step Solution
There are 3 Steps involved in it
Step: 1
In a bioreactor the heat loss rate is directly proportional to the cell growth rate To maintain the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


