Question
E. Calculations: 1. Use the table above and dilution formula ( M 1 V 1 = M 2 V 2 ), calculate the final concentration




E. Calculations:
1. Use the table above and dilution formula (M1V1 = M2V2), calculate the final concentration of [I- ] and [S2O32] for each trial separately.
2. Use the time and find the reaction rate (1/time).
3. Make a table of 4 trials with [I- ], [S2O32] and reaction rate (1/time).
4. Use the theoretical rate law (Rate = k . [I- ]x . [S2O32]y) and the data table you will be creating from steps 1-3. Find x by using [I- ] concentration change and reaction rate in two trials while keeping the concentration of [S2O32] is constant. This should give you the x. Then repeat the same thing with another set of two trials in which this time [I- ] is constant, but concentration [S2O32] is changing. This should give you y.
5. After finding x and y, you can now write the actual rate law of the reaction and find the orders in terms of [S2O32] concentration and [I- ] concentration as well as the overall reaction rate order.
6. By using one of the trials from your data table, you can now calculate the rate constant (k) for the reaction.
G. % Yield Calculation: (N.A.)
H. Reaction Scheme and/or Structure(s) and Name(s) of the Each Structure: (N.A.)
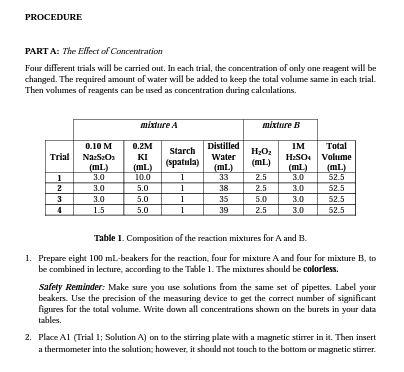
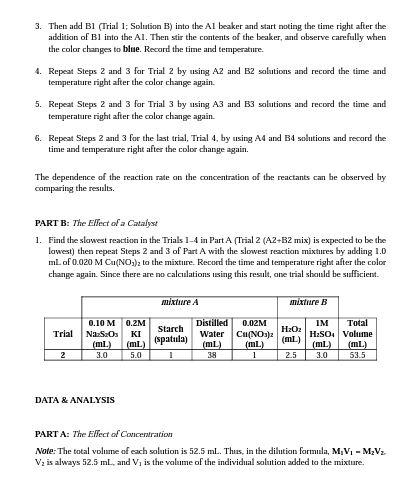
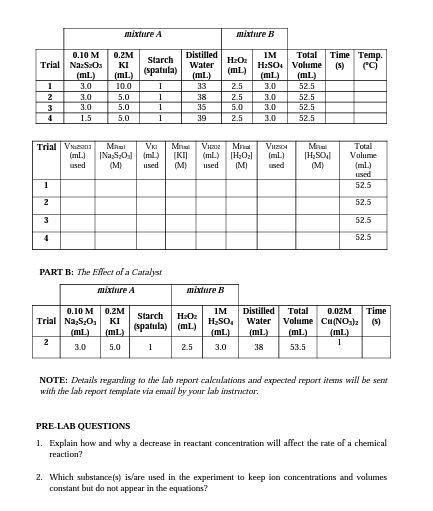
PURPOSE To determine the effects of concentration of reactants and catalyst an the reaction rates. INTRODUCTION The "dluck reaction" is a famous reaction for its dramatic colorless-to-blue color change and is aften uxed in chemistry courses to explore the rate at which renctions take place. The calar change occurs when l2 reacts with slarch to form a dark blue iodine/starch complex. The ability ta record the time at which the blue complex appears allaws the rate of reaction to be determined accurately with a stopwatch. In this experiment, the mite law for a reaction is determined using the method of initial rates. The effect of concentration on the rate of this reaction is determined by meesuring the initial reaction mate at several reactent concentrations. You will also examine the effect of a metal ion catalyst on the reaction rate. Lastly, you will investigate the effect of temperature an the rate of this reaction, which will allow you to determine the activation energy. The Clock Reaction The primary reaction to be studied is the axidation of by S2O22 (thiosulfate) in aqueous solution: 2I(aq) +H2O2(2q)+H+(2q) I2(2q)+2H2O (slowi) Equation 1 ladide hydragen bydrogen Indine water peroulde ion This reaction will be run in the presence of a known amount of S2Os2 (thiosulfate), which reacts very rapidly with L. As long 23SNO32 is present, I is consumed by SOO2 as fast as it is formed. This competing reaction prevents the I2 prodused from our reaction of interest from reacting with sarch, so no color change is abserved until the thiassulfate is completely used up. The "clack:" reaction is the reaction of a very small amount of S2O32 (thiosulfate) with the I2 prodiced in the primary reaction: I2 (2q) +2S2O32(aq)2I(aq) +S4O62 (aq) (fast) Equation 2 sadine thlosulfave lodide tetrathanate Jon The "clock" reaction will signal when the primary reaction forms a specific amount of 12. The amount of L formed before the color change can be calculated from the known amount of SOO22 addeed using the molar ratio in Equation 2. To find the rate of Equation 1, the change in the cancentration of I2 is monitared aver time. Belaw, [L2] is the change in the concentration of I2. and At represents the change in time: I2+(C6H10O5)nH2O blise colored complex Equintion 3 sadine starch The iodine (I2). produced in (Equation 1) is absorhed immediately by thiosulfate (S2O322) ion in the solution (Equation 2). When S2O32 is used up completely the concentration of I2 is increased, which can easily be abserved by the formation of deep blae color (Equnation 3). The rate expression for this reaction can be written as: R=k[I]x[H2O2]y[S2O32]x As soon as all the S2Os2 ions have reacted, the la still being forneed starts to accumulate and reacts with starch. Starch serves as an indicator to help us "see" the L, since the interaction between starch and I2 forms a blue starch-iodine complex. Thus, "At" is simply the time elapsed between mixing the reagents and the appearance of the blue color. Beciuse the 5S2O32 ion concentration in the reaction mixture is known, you can calculate " [12] using the stoichiometry of the "clock" reaction. Since the same amount of S2O22 is added to each run, [ I2] is also the same for each run. However, the amount of time for the appearance of the blue color varies with initial reaclant cancentrations, with temperature, and in the presence of catalyst, so At is not constant. Notes Regarding the Initial Concentrations, Dilation Formula, and Ion Molarities: The initial concentrations of reactants are calculated for the moment at which they are mixed. At that time, the solutions have mutally diluted each other (raised the volume of total salution, with or without adding moles of the solute), but have not yet started disappearing from solution (via reaction). For each ion in solution, a new molarity must be calculated that takes inta consideration the new total volume of the salution, and the other ions that were added. The concentrations and volumes of the reactants are given in Table 1. To determine the concentration of an ian in solution, consider the stoichiometric relationship between the ianic compound and the number of ions formed in solution. For example, a 0.20M solution of KI releses 0.20MJ5 and 0.20MK ions. The situation wauld be different if the source of I was Cal2, since 2 moles of T ians would be released for each mole of Cal2 that dissociates. Calculate the concentration of each reactant after combining the solutions, but before the chemical reaction begins. Note- The tatal volume of each solution is 52.5ml (See Table 1). Thus, in the dilution formula, M1V1=M2V2,V2 is always 52.5mL, and V1 is the volume of the individaal solution added to the mixire. Preliminary Calculations Involving the "Clock" Reaction Using the dilution formula, the concentration of S2Os2 in the mixture is 1.2101M. According to the stoichiometry of the clock reaction in Equation 2, the number of molles of I2 is one half the number of moles of S2O32. The blue calar will appear when 6.0104M1I2 (with two significant figares) has been formed by the primary reaction. This number remains constant in all Runs, and sD provides A[I] in all of rate calculations. For all nuns: 4[I2]=6.0104MI2 Note- When the solution turns blue, only a very small percentage of the reactants have been used up. Thus, the initial concentrations of reactants can be used in the rate law with very little error. Typically, the error is less than 5%. Reaction Rate The rate law for Equation 1 will be determined by measuring the initial rate of reaction with varying initial reactant concentrations. The concentration of S3O32 in the reaction mixture is very small compared to the other reactants present, such that the measured rate is the initial rate of the reactian. The rate law for Equation 1 (SLOW STEP is given below: Rate=k[I]x[SaO2]yEquation4 In Equation 4,k is the rate constant, and x and y represent the order af Iand S2O32, respectively. These valus will experimentally be determined from the collected data. Effect of a Catalyst on the Reaction Rate A comparison of the reaction rate with and without a catalyst will demonstrate catalytic action. The Cu2+ ion, added in the form of a dillute Ca( NO1)z solution, makes a suatsble catalyst. PART A: The Elfect of Concentration Faur different traials will be carried out. In each trial, the concentration of only one reagent will be changed. The required amount of water will be added to keep the total volume same in each trial. Then volumes of reagents can be used as concentration during calculations. Table 1. Composition of the reaction mixhres for A and B. 1. Prepare eight 100mL-beakers for the reaction, four for mixture A and four for mixtare B, to be combined in lecture, accotring to the Table 1 . The mixtures should be colorless. Safety Reminder: Make sure you use solutions from the same set of pipettes. Label your beakers. Use the precision of the measuring device to get the correct number of significant figures for the total volume. Write dawn all concentrations shown an the barets in your data tables. 2. Place A1 (Trial 1; Solution A) on to the stirring plate with a magnetic stirrer in it. Then insert a thermometer into the solution; however, it should not touch to the bottom or magnetic stirrer. 3. Then add B1 (Trial 1; Solation B) into the A1 beaker and start noting the time right after the addition of B1 into the Al. Then stir the contents of the beaker, and observe carefully when the color changes to blue. Recard the time and temperature. 4. Repeat 5 teps 2 and 3 for Trial 2 by using A2 and B2 solutions and recond the time and temperature right after the color change again. 5. Repeat Steps 2 and 3 for Trial 3 by using A3 and B3 solutions and record the time and temperature right after the color change again. 6. Repeat 5 teps 2 and 3 for the last trial, Trial 4 , by using A4 and B4 solutions and record the time and temperature right after the color change again. The dependence of the reaction rate on the concentration of the reactants can be abserved by comparing the results. PART B: The Ellect of a Citalyst 1. Find the slawest reaction in the Trials 14 in Part A (Trial 2(A2+B2mix) is expected to be the lawest) then repeat Steps 2 and 3 of Part A with the slowest reaction mixtures by adding 1.0 mL of 0.020MCa(NO3)2 to the mixture. Record the time and temperature right after the color change again. Since there are no calcalations using this result, ane trial should be sufficient. DATA \& ANALYSIS PART A: The Emect of Concentration Note- The total volume of each solution is 52.5mL. Thus, in the dilution formula, M1V1=M2V2. V2 is always 52.5mL, and V1 is the volame of the individual salution added to the mixture. PART B: The ERfect of a Catalyst NOTE: Details regarding to the Iab report calculations and expected repont items will be sert with the lab repovt femplate via email by your lab instructor. PRE-LAB QUESTIONS 1. Eixplain haw and why a decrease in reactant concentration will affect the rate of a chemical reaction? 2. Which substance(s) is/are used in the experiment to keep ion concentrations and volumes constant but do not appear in the equations? 3. What ion must be used up (limiting reactant) in the clock reaction to cause the solution to turn blise? 4. If 5 drops of 0.15MKI are added to 40 drops of Naz2Sa2, what is the final concentration of KI ? (20 draps =1mL) 5. Which chemical acts as a catalyst in this experiment? 6. What is the rale of starch in the experiment? 7. What variablesifactors during the experiment will affect yaur results? 8. What are the safety concerns associated with this experiment? POST-LAB QUESTIONS 1. Explain how and why the reaction rate changes as concentrations of the reactants change. 2. Explain the general calculation procedure used to salve for reactant orters in the rate law. How did you determine which runs to use to solve for the order of each reartant? 3. Compare your avernge reaction time for the trial with and without Cu(NO3)2 to your reaction. Haw did the addition of Cu(NO2)2 affect the rate of reaction? WHY did Ca(NO3)2 have this 4. Discuss at least 2 sources of error - haw they affected yaur results and how you would carrect ther if you were to repeat the experiment
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


