Answered step by step
Verified Expert Solution
Question
1 Approved Answer
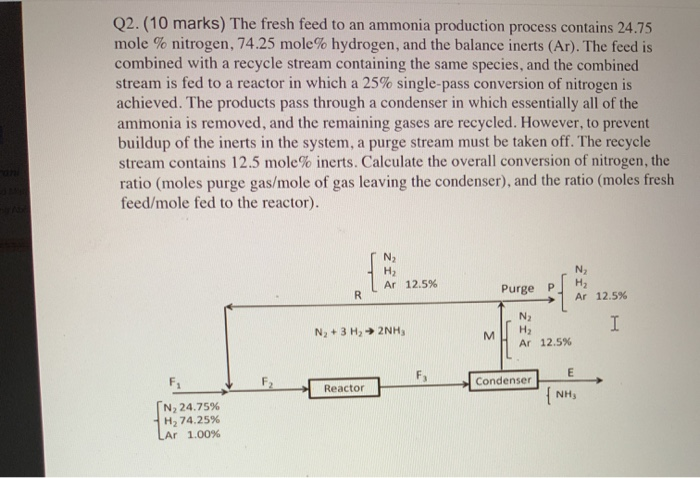
e fresh feed to an ammonia production process contains 2 4 . 7 5 mole % nitrogen, 7 4 . 2 5 mole % hydrogen,
e fresh feed to an ammonia production process contains mole nitrogen, mole hydrogen, and thebalance inerts I The feed is combined with a recycle stream containing the same species, and the combined stream isfed to a reactor in which a singlepass conversion of nitrogen is achieved. The products pass through a condenser inwhich essentially all of the ammonia is removed, and the remaining gases are recycled. However, to prevent the buildupof the inerts in the system, a purge stream must be taken off. The recycle stream contains mole inerts. Calculate theoverall conversion of nitrogen, the ratio moles purge gasmole of gas leaving the condenser and the ratio moles freshfeedmole fed to the reactor
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


