Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) (i) (b) disappearance of A is given by the equation: (ii) A undergoes two simultaneous reactions to produce B and C according to
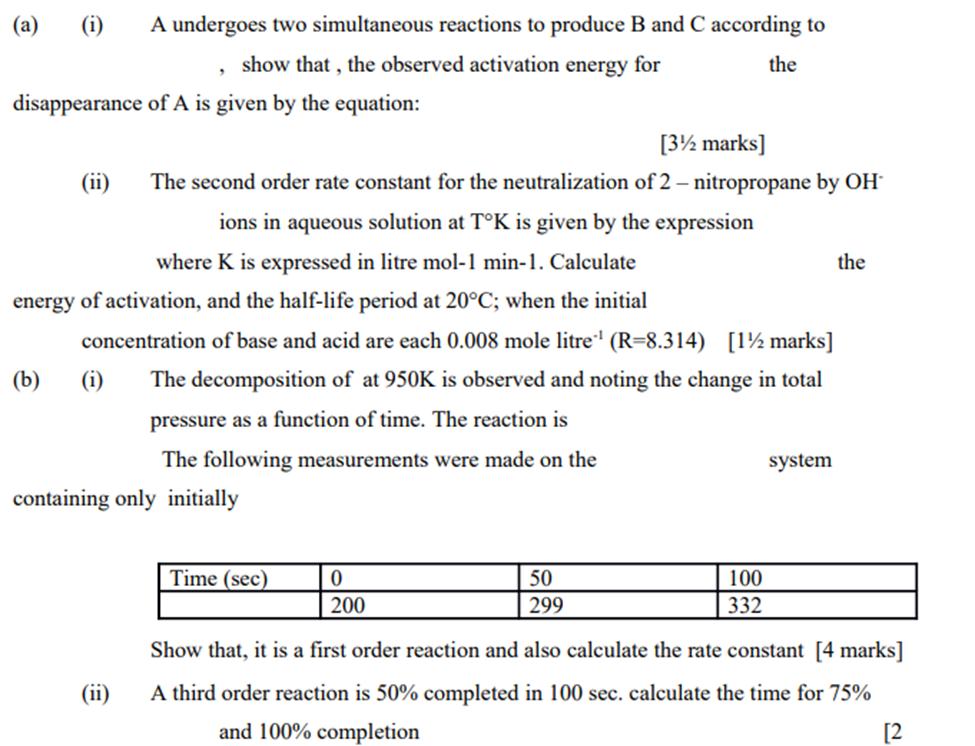
(a) (i) (b) disappearance of A is given by the equation: (ii) A undergoes two simultaneous reactions to produce B and C according to show that, the observed activation energy for the (i) . where K is expressed in litre mol-1 min-1. Calculate energy of activation, and the half-life period at 20C; when the initial concentration of base and acid are each 0.008 mole litre (R-8.314) [1 marks] The decomposition of at 950K is observed and noting the change in total pressure as a function of time. The reaction is The following measurements were made on the (ii) [3 marks] The second order rate constant for the neutralization of 2- nitropropane by OH ions in aqueous solution at TK is given by the expression containing only initially Time (sec) 0 200 50 299 100 332 system the Show that, it is a first order reaction and also calculate the rate constant [4 marks] A third order reaction is 50% completed in 100 sec. calculate the time for 75% and 100% completion [2
Step by Step Solution
★★★★★
3.51 Rating (171 Votes )
There are 3 Steps involved in it
Step: 1
Question a part 1 A undergoes two simultaneous reactions to produce B and C according to the following equation A B C The observed activation energy for the disappearance of A is given by the equation ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


