Question: ECH3854 - Engineering Computations - Spring 2020 > Consecutive Calculations - Chemical Engineering > Peng-Robinson equation of state O solutions submitted (max: Unlimited) The Peng-Robinson
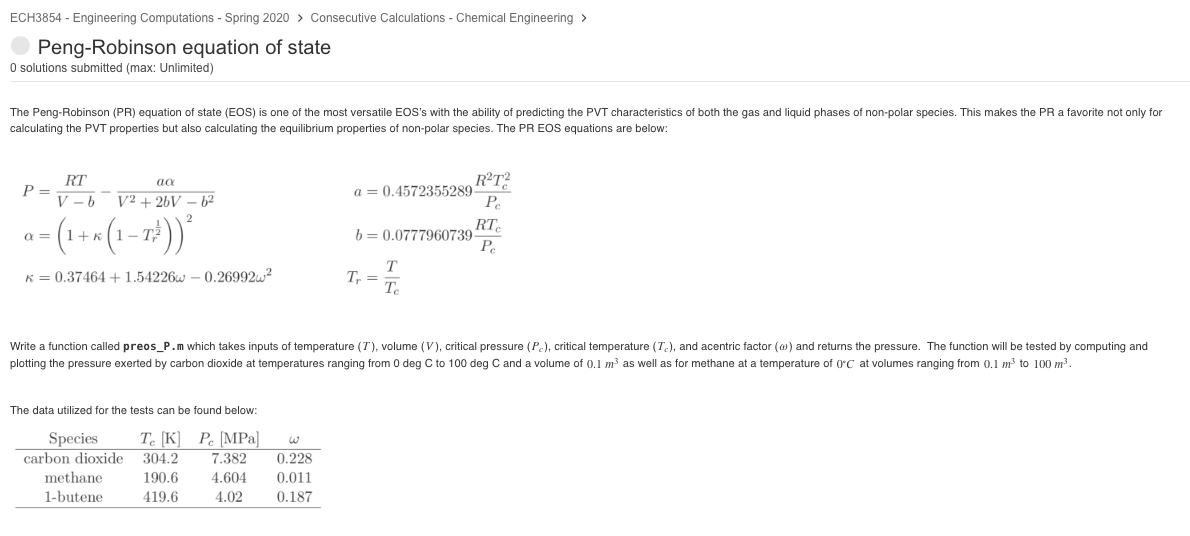

ECH3854 - Engineering Computations - Spring 2020 > Consecutive Calculations - Chemical Engineering > Peng-Robinson equation of state O solutions submitted (max: Unlimited) The Peng-Robinson (PR) equation of state (EOS) is one of the most versatile EOS's with the ability of predicting the PVT characteristics of both the gas and liquid phases of non-polar species. This makes the PR a favorite not only for calculating the PVT properties but also calculating the equilibrium properties of non-polar species. The PR EOS equations are below: a = 0.4572355289 RT? Pe RT aa Pev - V2 + 26V 62 a= (1+x (1-) K = 0.37464 +1.54226 - 0.269922 b = 0.0777960739P RT Tr = Write a function called preos_P.m which takes inputs of temperature (T), volume (V), critical pressure (Pc), critical temperature (Tc), and acentric factor (w) and returns the pressure. The function will be tested by computing and plotting the pressure exerted by carbon dioxide at temperatures ranging from 0 deg C to 100 deg C and a volume of 0.1 m as well as for methane at a temperature of 0C at volumes ranging from 0.1 m to 100 m2 The data utilized for the tests can be found below: Species carbon dioxide methane 1-butene T K 304.2 190.6 419.6 P MPa 7.382 4.604 4.02 W 0.228 0.011 0.187 Code to call your function 1 % common to all 2 R = 8.314; $ (m^3*Pa/mol/K] 4 % for CO2 T ramp, fixed V 5 T = [0:100]' + 273.15; $ [K] 6 V = 0.1; %m3 8 % CO2 9 PC_CO2 = 7.382e6; [Pa] 10 Tc_CO2 = 304.2; % (K) 11 omega_CO2 = 0.228; 12 P_CO2 = preos_P(T, V, PC_CO2, Tc_C02, omega_CO2, R); 14 % plot P_CO2 vs T 15 figure 16 plot(T-273.15, P_CO2) 17 xlabel('Temperature (^\circc]') 18 ylabel('Pressure [Pa]') 19 title('p from Peng-Robinson EOS for Co_2') 20 grid on $ for CH4 V ramp, fixed T 23 T = 6 + 273.15; 24 V = logspace(-1, 2); [K] m^3 26 % CH4 27 Pc_CH4 = 4.604e6; % [Pa] 28 TC_CH4 = 190.6; $ [K] 29 omega_CH4 = 0.011; 30 P_CH4 = preos_P(T, V, PC_CH4, TC_CH4, omega_CH4, R); 34 32 % plot P_CH4 vs V figure plot(V, P_CH4) xlabel( 'Volume [m^3]') 36 ylabel('Pressure [Pa]') 37 title('p from Peng-Robinson EOS for CH_4') 38 grid on
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


