Answered step by step
Verified Expert Solution
Question
1 Approved Answer
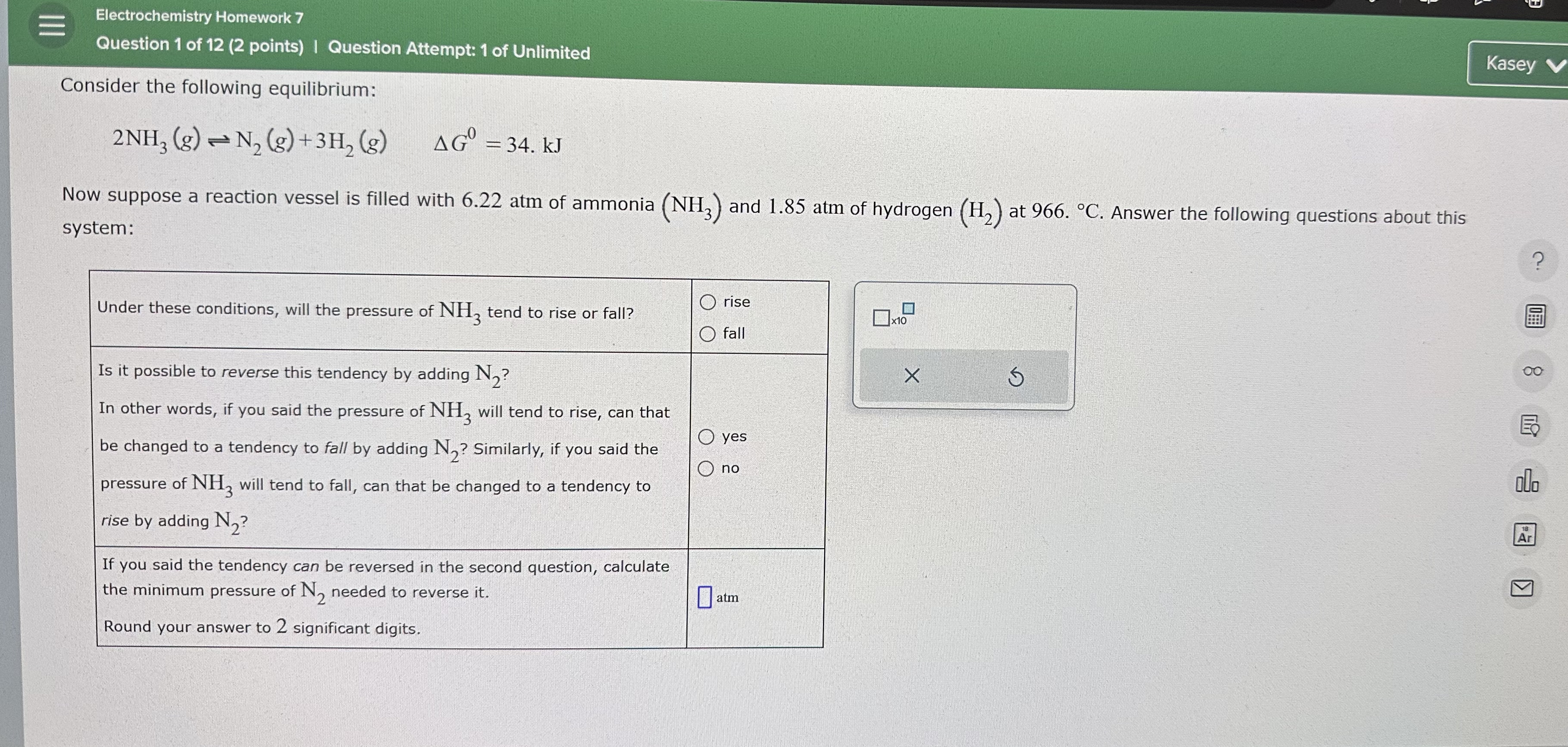
Electrochemistry Homework 7 Question 1 of 1 2 ( 2 points ) I Question Attempt: 1 of Unlimited Consider the following equilibrium: 2 N H
Electrochemistry Homework
Question of points I Question Attempt: of Unlimited
Consider the following equilibrium:
Now suppose a reaction vessel is filled with atm of ammonia and atm of hydrogen at Answer the following questions about this system:
tableUnder these conditions, will the pressure of tend to rise or fall?,tablerisefallIs it possible to reverse this tendency by adding In other words, if you said the pressure of will tend to rise, can that,be changed to a tendency to fall by adding Similarly, if you said the,pressure of will tend to fall, can that be changed to a tendency torise by adding tableIf you said the tendency can be reversed in the second question, calculatethe minimum pressure of needed to reverse itRound your answer to significant digits.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


