Answered step by step
Verified Expert Solution
Question
1 Approved Answer
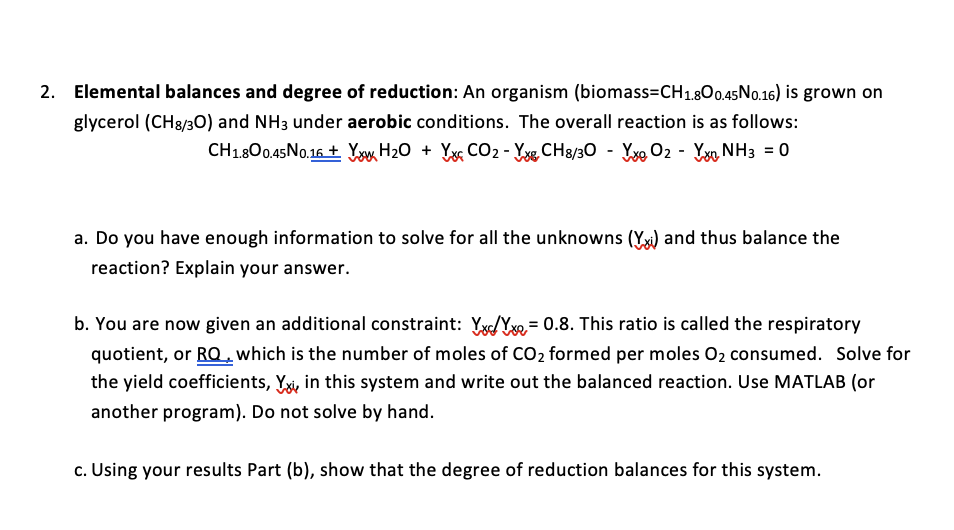
Elemental balances and degree of reduction: An organism ( biomass = C H 1 . 8 O 0 . 4 5 N 0 . 1
Elemental balances and degree of reduction: An organism biomass is grown on glycerol and under aerobic conditions. The overall reaction is as follows:
a Do you have enough information to solve for all the unknowns and thus balance the reaction? Explain your answer.
b You are now given an additional constraint: This ratio is called the respiratory quotient, or which is the number of moles of formed per moles consumed. Solve for the yield coefficients, in this system and write out the balanced reaction. Use MATLAB or another program Do not solve by hand.
c Using your results Part b show that the degree of reduction balances for this system.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


