Question
Elements that appear in the same column of the periodic table often share similar chemical properties. In the case of the alkaline earth metals, this
Elements that appear in the same column of the periodic table often share similar chemical properties. In the case of the alkaline earth metals, this is troublesome since the body treats calcium (necessary for proper bone growth) and radium (a radioactive element) as chemically similar, storing both in the bone marrow. The radium then bombards nearby bone cells with alpha particles, causing them to "crumble." Radium poisoning investigations often center on the identification of radium and its isotopes in bone samples using a mass spectrometer. Pictured is a schematic of a simplified mass spectrometer, showing the paths of calcium, barium (another alkaline earth metal), and radium isotopes entering the chamber. The region shown is immersed in a constant magnetic field of 0.552 T pointing out of the plane of the schematic. The motion of the positively charged isotopes toward the right was initiated by a potential difference of 2979 V on the two plates shown. Using the data shown in the table below, calculate the path radius of the Ca ion.
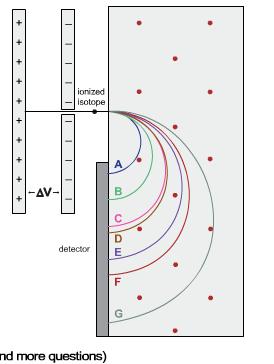
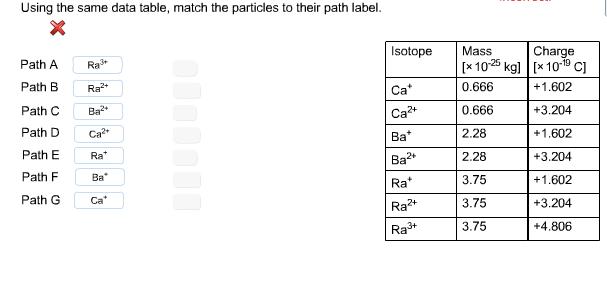
ionized Isotope A -AV- B. D detector E G nd more questions)
Step by Step Solution
3.42 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


