Answered step by step
Verified Expert Solution
Question
1 Approved Answer
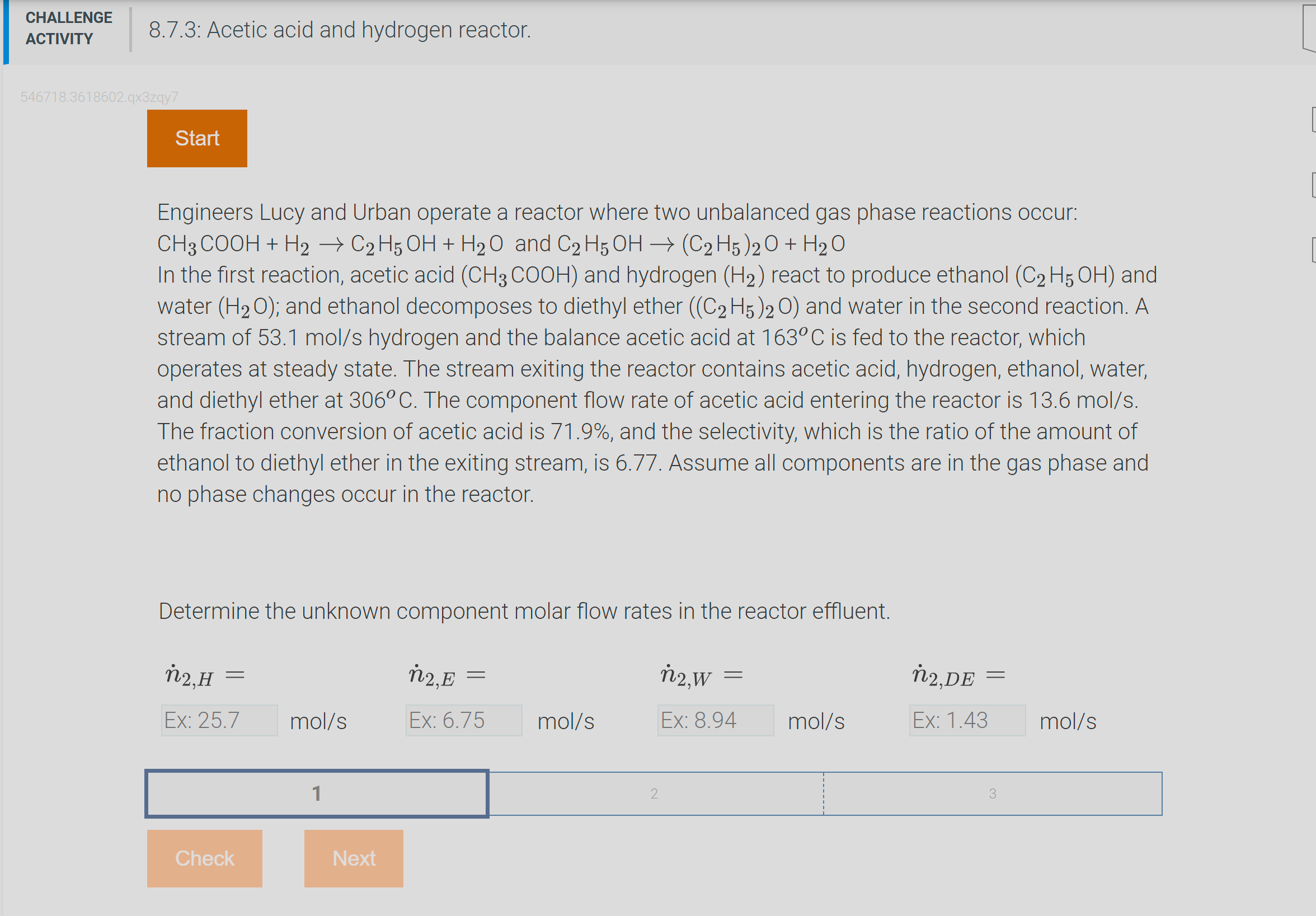
Engineers Lucy and Urban operate a reactor where two unbalanced gas phase reactions occur: C H 3 C O O H + H 2 C
Engineers Lucy and Urban operate a reactor where two unbalanced gas phase reactions occur:
and
In the first reaction, acetic acid and hydrogen react to produce ethanol and
water ; and ethanol decomposes to diethyl ether and water in the second reaction. A
stream of hydrogen and the balance acetic acid at is fed to the reactor, which
operates at steady state. The stream exiting the reactor contains acetic acid, hydrogen, ethanol, water,
and diethyl ether at The component flow rate of acetic acid entering the reactor is
The fraction conversion of acetic acid is and the selectivity, which is the ratio of the amount of
ethanol to diethyl ether in the exiting stream, is Assume all components are in the gas phase and
no phase changes occur in the reactor.
Determine the unknown component molar flow rates in the reactor effluent.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


