Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Enzymes are important molecules in biochemistry that catalyze reactions. The energy diagram illustrates the difference between a catalyzed reaction and an uncatalyzed reaction. Label
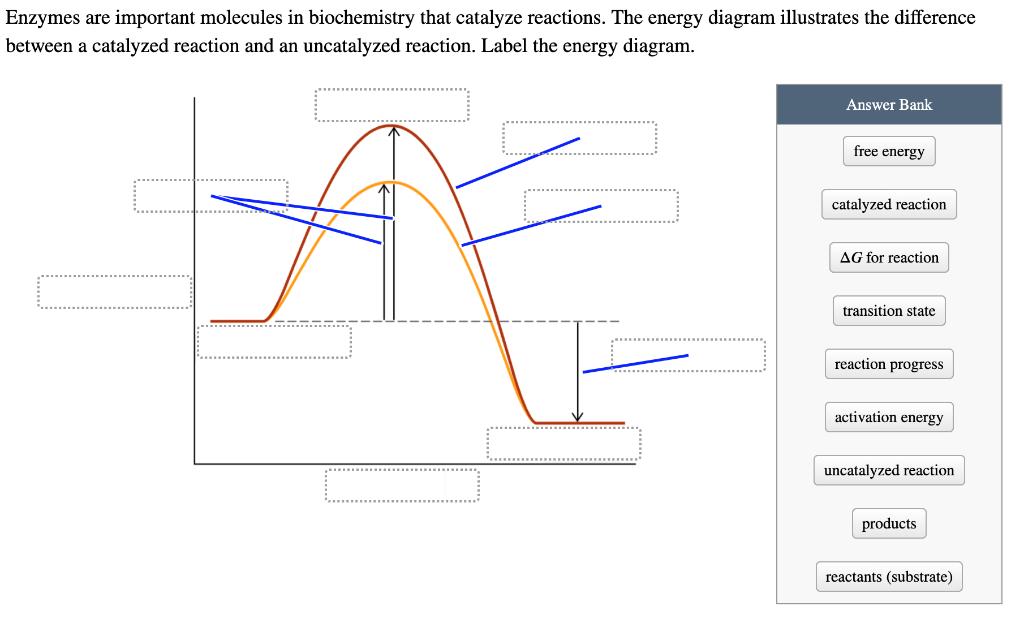

Enzymes are important molecules in biochemistry that catalyze reactions. The energy diagram illustrates the difference between a catalyzed reaction and an uncatalyzed reaction. Label the energy diagram. Answer Bank free energy catalyzed reaction AG for reaction transition state reaction progress activation energy uncatalyzed reaction products reactants (substrate) What ways do enzymatic catalysts increase the rates of reactions? They increase the concentration of reactants. They promote the formation of a transition state. They decrease the free energy of the reaction. They lower the activation energy of the reaction. They shift the reaction equilibrium toward the products.
Step by Step Solution
★★★★★
3.37 Rating (144 Votes )
There are 3 Steps involved in it
Step: 1
AG for reaction Enzymes are important molecules in biochemistry t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


