Answered step by step
Verified Expert Solution
Question
1 Approved Answer
es Mailings Review View me what yo Help QUESTIONS Q-1. Tennis balls are usually filled with either air or N2 gas toa pressure above atmospheric
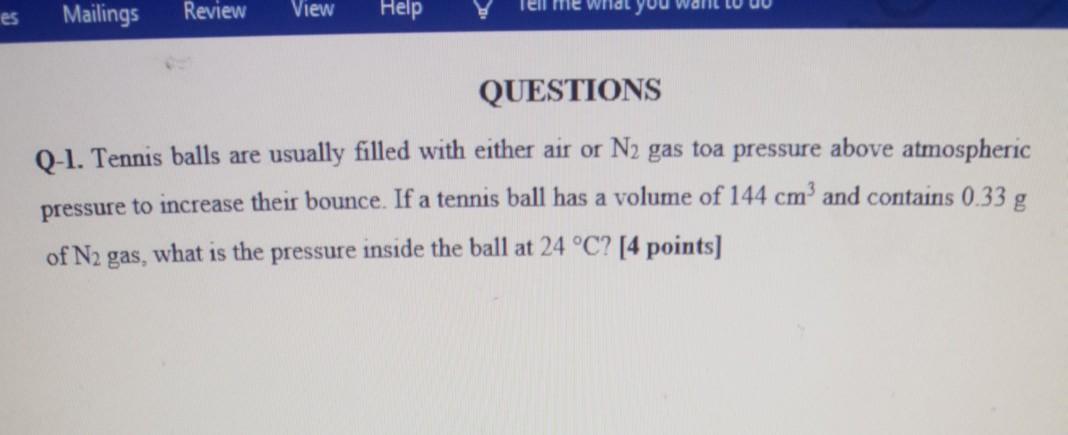






es Mailings Review View me what yo Help QUESTIONS Q-1. Tennis balls are usually filled with either air or N2 gas toa pressure above atmospheric pressure to increase their bounce. If a tennis ball has a volume of 144 cm' and contains 0.33 g of N2 gas, what is the pressure inside the ball at 24 C? [4 points) Q-2. In the first step of the industrial process for making nitric acid, ammonia reacts with oxygen in the presence of a suitable catalyst to form nitric oxide and water vapor. [4 points] 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g) How many liters of NH3(g) at 850 C and 506.6 kPa are required to react with 1.00 mol of O2(g) in this reaction? Q-3. Which of the following bonds is most polar: SCI, S-Br, Se-Cl, or Se-Br? [2 points] Q 4. Draw the Lewis structure for (a) NO-ion, (b) CH. (C) CIO. (d) PO.. [8 points) Q-5. The cyanate ion NCO, has three possible Lewis structures. [6 points] (a) Draw these three structures and assign formal charges in each. (b) Which Lewis structure is dominant? Q 6. Phosgene, a substance used in poisonous gas warfare during World War I, is so named because it was first prepared by the action of sunlight on a mixture of carbon monoxide and chlorine gases. Its name comes from the Greek words phos (light) and genes (bom of). Phosgene has the following elemental composition: 12.14% C, 16.17% 0, and 71.69% Cl by mass. Its molar mass is 98.9 g/mol. [12 points) (a) Determine the molecular formula of this compound (b) Draw three Lewis structures for the molecule that satisfy the octet rule for each atom. (The Cl and O atoms bond to C.) (c) Using formal charges, determine which Lewis structure is the dominant one. (d) Using average bond enthalpies, estimate H for the formation of gaseous phosgene from CO(g) and Ch(g) Q-7. Which has the lowest first ionization energy, B, Al, C, or Si? Which has the highest? (3 points] Q-8. Write the electron configurations for (a) Ga3+, (b) Cr3+, and (c) Br - [6 points] Q-9. Use the periodic table to write the condensed electron configuration for [4 points] (a) Co (element 27). (b) In (element 49) Q-10. Boron, atomic number 5, occurs naturally as two isotopes, 1B and B, with natural abundances of 19.9% and 80.1%, respectively. [18 points] (a) In what ways do the two isotopes differ from each other? Does the electronic configuration of 10 differ from that of IB? (b) Draw the orbital diagram for an atom of 'B. Which electrons are the valence electrons? (c) Indicate three ways in which the Is electrons in boron differ from its 2s electrons. (d) Elemental boron reacts with fluorine to form BF3, a gas. Write a balanced chemical equation for the reaction of solid boron with fluorine gas. (e) AH?" for BF3(g) is -1135.6 kj/mol. Calculate the standard enthalpy change in the reaction of boron with fluorine. (1) Will the mass percentage of F be the same in 19BF3 and 1lBF3? If not, why is that the case? Q-11. A 0.5865-g sample of lactic acid (HC3H503) reacts with oxygen in a calorimeter whose heat capacity is 4.812 kj/C. The temperature increases from 23.10 to 24.95 C. Calculate the heat of combustion of lactic acid [6 points] (a) per gram (b) per mole
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


