Question
Esters (i) aliphatic: RCOR' M+ weak but observable note: M+ gets less intense as the alcohol portion ( ) increases in size: e.g. C4
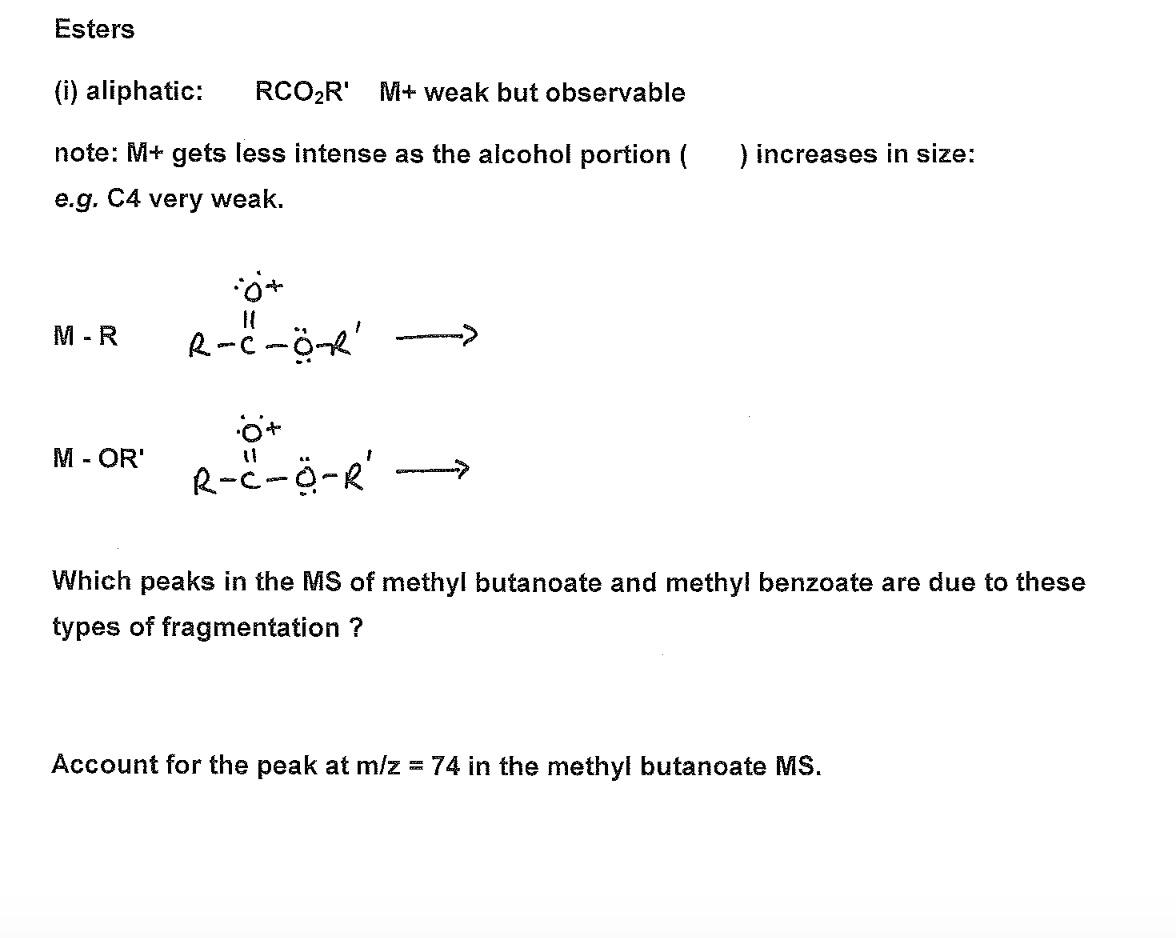
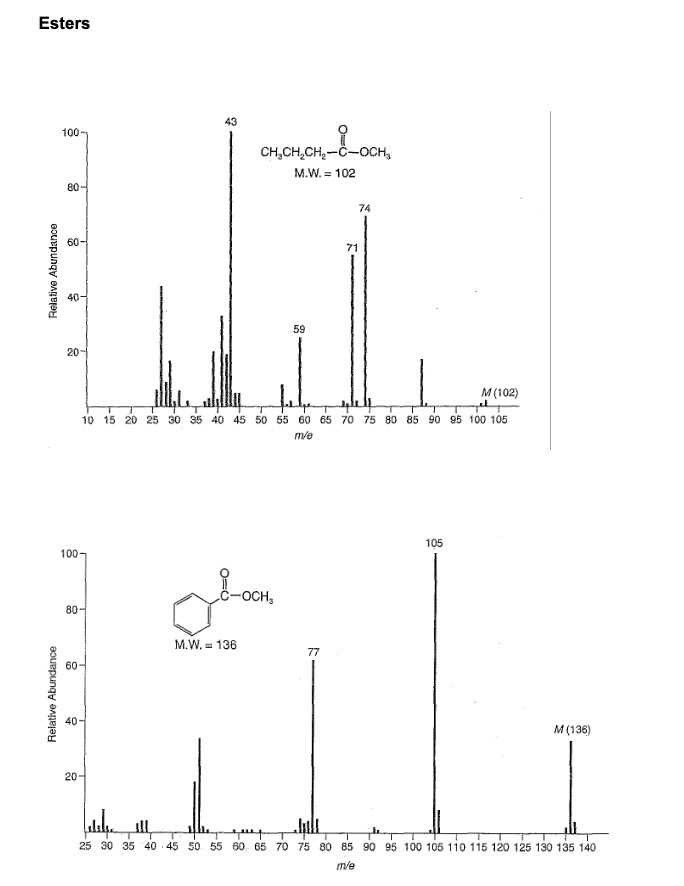
Esters (i) aliphatic: RCOR' M+ weak but observable note: M+ gets less intense as the alcohol portion ( ) increases in size: e.g. C4 very weak. M-R M - OR' 0+ R-C-O-R + R-C-O-R' Which peaks in the MS of methyl butanoate and methyl benzoate are due to these types of fragmentation ? Account for the peak at m/z = 74 in the methyl butanoate MS. Esters Relative Abundance Relative Abundance 100 80 60- 40- 20 100- 80- 60 40 43 20- CHCHCH-C-OCH M.W. = 102 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 105 m/e 3-com. M.W. 136 59 71 77 74 M (102) 105 M (136) elekea ill T quep T 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 105 110 115 120 125 130 135 140 m/e
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Fragmentation pat...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics Alive
Authors: Wendy J. Steinberg
2nd Edition
1412979501, 978-1483343341, 1483343340, 978-1412979504
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



