Question
Ethane (C2H6) enters a reactor shown in the figure at a temperature of 25C and a pressure of 100 kPa; It burns completely with
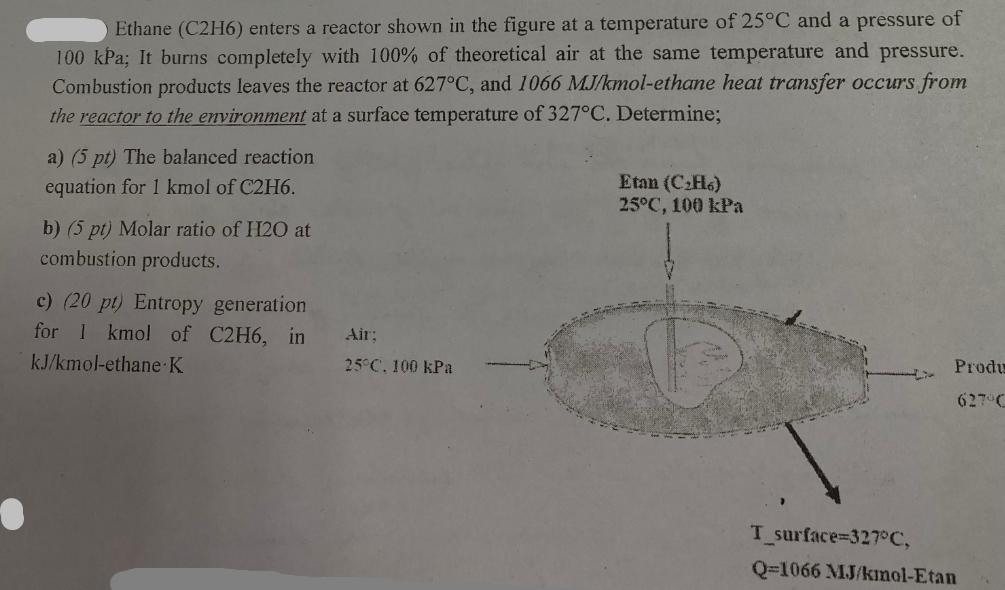
Ethane (C2H6) enters a reactor shown in the figure at a temperature of 25C and a pressure of 100 kPa; It burns completely with 100% of theoretical air at the same temperature and pressure. Combustion products leaves the reactor at 627C, and 1066 MJ/kmol-ethane heat transfer occurs from the reactor to the environment at a surface temperature of 327C. Determine; a) (5 pt) The balanced reaction equation for 1 kmol of C2H6. b) (5 pt) Molar ratio of H20 at combustion products. c) (20 pt) Entropy generation for 1 kmol of C2H6, in kJ/kmol-ethane K Air; 25C. 100 kPa Etan (CH6) 25C, 100 kPa T_surface=327C, Produ 627 C Q-1066 MJ/kmol-Etan
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



