Question
Ethanol is produced from ethylene and water according to the reaction: [ mathrm{C}_{2} mathrm{H}_{4}+mathrm{H}_{2} mathrm{O} ightarrow mathrm{C}_{2} mathrm{H}_{5} mathrm{OH} ] In the process shown below,
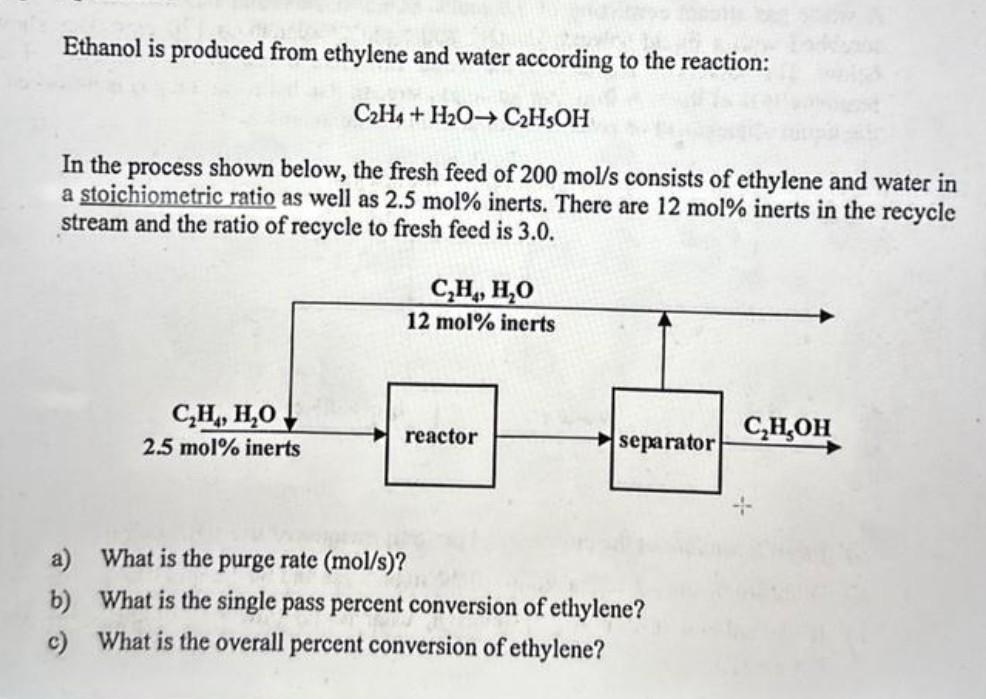
Ethanol is produced from ethylene and water according to the reaction: \[ \mathrm{C}_{2} \mathrm{H}_{4}+\mathrm{H}_{2} \mathrm{O} ightarrow \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH} \] In the process shown below, the fresh feed of200 mol/sconsists of ethylene and water in a stoichiometric ratio as well as2.5 mol%inerts. There are12 mol%inerts in the recycle stream and the ratio of recycle to fresh feed is3.0. a) What is the purge rate(mol/s)? b) What is the single pass percent conversion of ethylene? c) What is the overall percent conversion of ethylene?
Ethanol is produced from ethylene and water according to the reaction: C2H4+H2OC2H5OH In the process shown below, the fresh feed of 200mol/s consists of ethylene and water in a stoichiometric ratio as well as 2.5mol% inerts. There are 12mol% inerts in the recycle stream and the ratio of recycle to fresh feed is 3.0. a) What is the purge rate (mol/s) ? b) What is the single pass percent conversion of ethylene? c) What is the overall percent conversion of ethyleneStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


