Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Ethylene and chlorine ( streams 1 and 2 ) are fed in stoichiometric amounts at 2 5 C and 1 atm to a 1 .
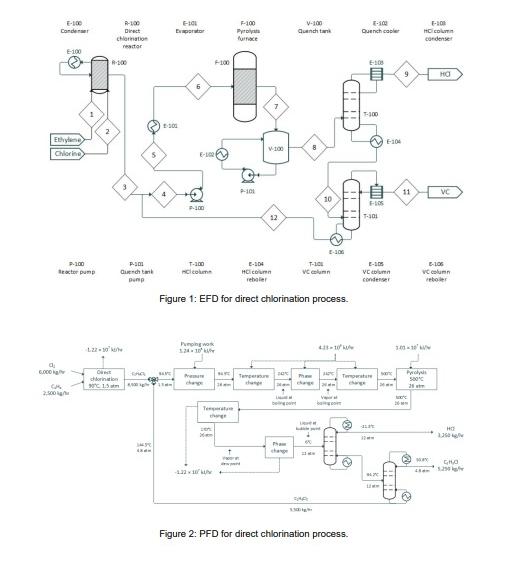
Ethylene and chlorine streams and are fed in stoichiometric amounts at and atm to a atm bubble column reactor that circulates the reactant gases via sparger dispersion through liquid dichloroethane that is maintained at by a coldwater stream The bubble column reactor design maximizes ethylene mass transfer and its use in industry is wellcharacterized Orejas The product stream exiting the direct chlorination reactor is a pure dichloroethane stream stream with limited side reaction and unreacted feed compounds. R also contains ferric chloride that acts as a Lewis acid catalyst, polarizing chlorine to increase its electrophilic activity and encourage attack on ethylene's double bond Wachi & Asai, Kinetics of Dichloroethane Formation from Ethylene and Cupric Chloride,
The dichloroethane stream is joined by a recycle producing stream and fed through a centrifugal pump ; stream and a firedheat evaporator ; stream to achieve pressurization to atm, complete vaporization, and a temperature increase to This stream is flown into a pyrolysis furnace at the same temperature and pressure, where it is primarily converted to vinyl chloride. Although a catalytic pyrolysis method exists, similar conversion levels can be reached without a catalyst, making the noncatalytic approach a simpler, less expensive, and more favorable method Dreher Torkelson, & Beutel, ; Rus, The furnace contents are transferred to a quench tank ; stream
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


