Answered step by step
Verified Expert Solution
Question
1 Approved Answer
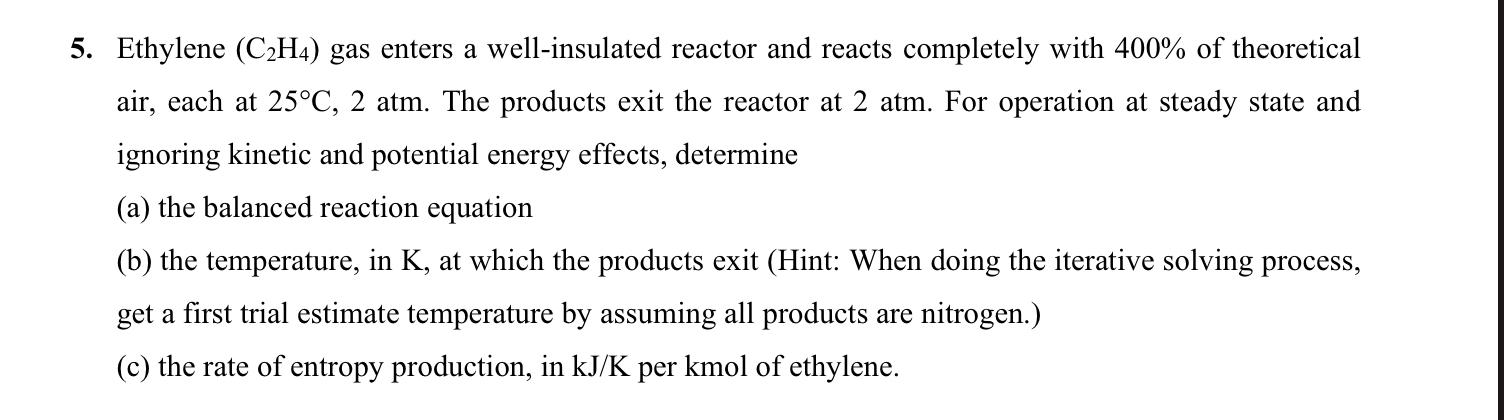
Ethylene ( C 2 H 4 ) gas enters a well - insulated reactor and reacts completely with 4 0 0 % of theoretical air,
Ethylene gas enters a wellinsulated reactor and reacts completely with of theoretical air, each at atm. The products exit the reactor at atm. For operation at steady state and ignoring kinetic and potential energy effects, determine
a the balanced reaction equation
b the temperature, in at which the products exit Hint: When doing the iterative solving process, get a first trial estimate temperature by assuming all products are nitrogen.
c the rate of entropy production, in per kmol of ethylene.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


