Answered step by step
Verified Expert Solution
Question
1 Approved Answer
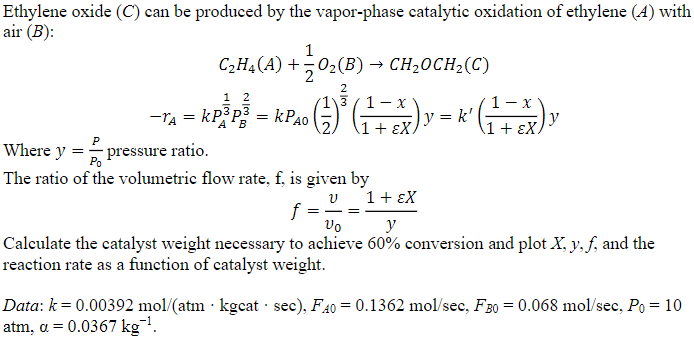
Ethylene oxide ( C ) can be produced by the vapor - phase catalytic oxidation of ethylene ( A ) with air ( B )
Ethylene oxide can be produced by the vaporphase catalytic oxidation of ethylene with
air :
Where pressure ratio.
The ratio of the volumetric flow rate, is given by
Calculate the catalyst weight necessary to achieve conversion and plot and the
reaction rate as a function of catalyst weight.
Data:
atm,
Hint: Use bisection method and the builtin ode solver in PYTHON.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


