Question
Ethylene oxide is formed by the partial oxidation of ethylene in the gas phase (see figure). In this process, 1000 mol/h of pure ethylene are
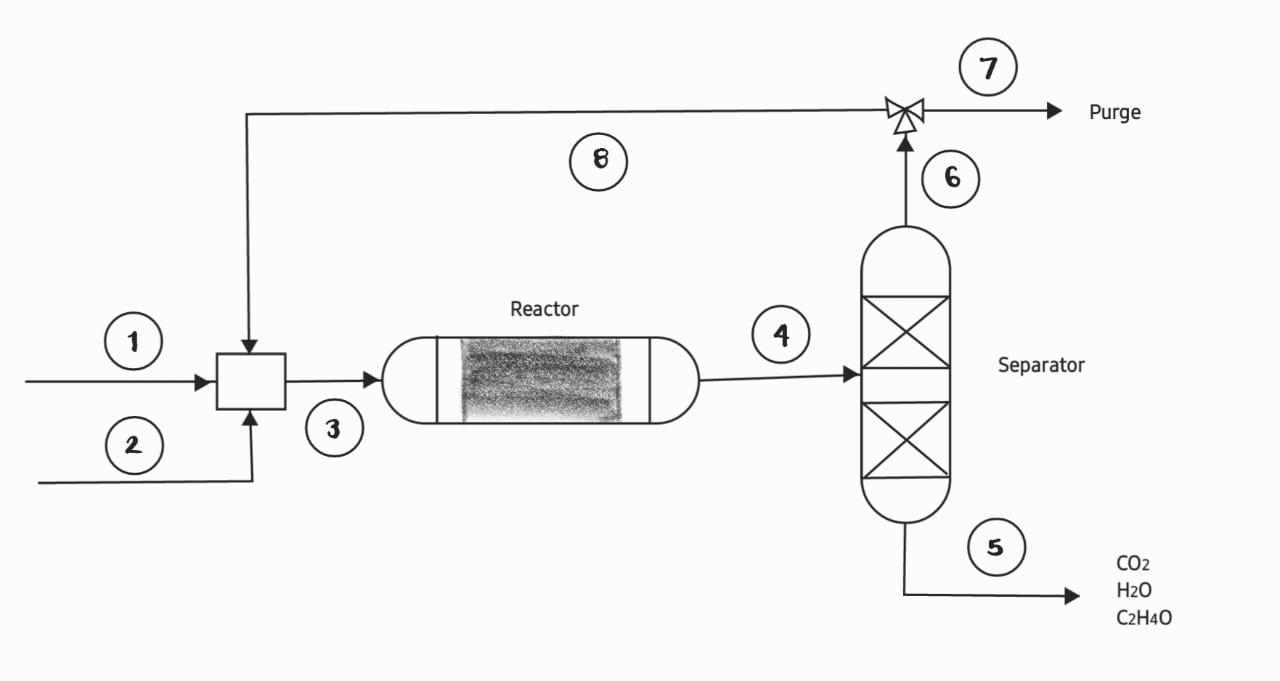
Ethylene oxide is formed by the partial oxidation of ethylene in the gas phase (see figure). In this process, 1000 mol/h of pure ethylene are fed into the system, this stream is mixed with air. The molar ratio between fresh ethylene and oxygen in the air is 2 to 1. The ethylene conversion in one pass is 25%. The stream leaving the reactor contains ethylene, ethylene oxide, water, carbon dioxide and nitrogen, all oxygen is consumed.
The effluent from the reactor is fed to a separation unit, in which the ethylene and nitrogen are removed from the other gases. A portion of the ethylene and nitrogen is purged and the recycle is mixed with the fresh ethylene and the air stream. The ethylene oxide, water and carbon dioxide are sent to another separation process. In stream (5) 50 mol/h of carbon dioxide are quantified. Reactions in the gas phase occur according to the following stoichiometry:
C2H4 + O CH4O
CH4 + 3 O2 2 HO + 2 CO
Determine all the unknowns of the problem. Explain each calculation in detail.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


