Answered step by step
Verified Expert Solution
Question
1 Approved Answer
EX) Find the pH,[OCl], and [HOCl] in a solution, containing 104M of sodium hypochlorite ( NaOCl), and 5104M of hypochlorous acid (HOClpKa=7.5) at 25C; neglect
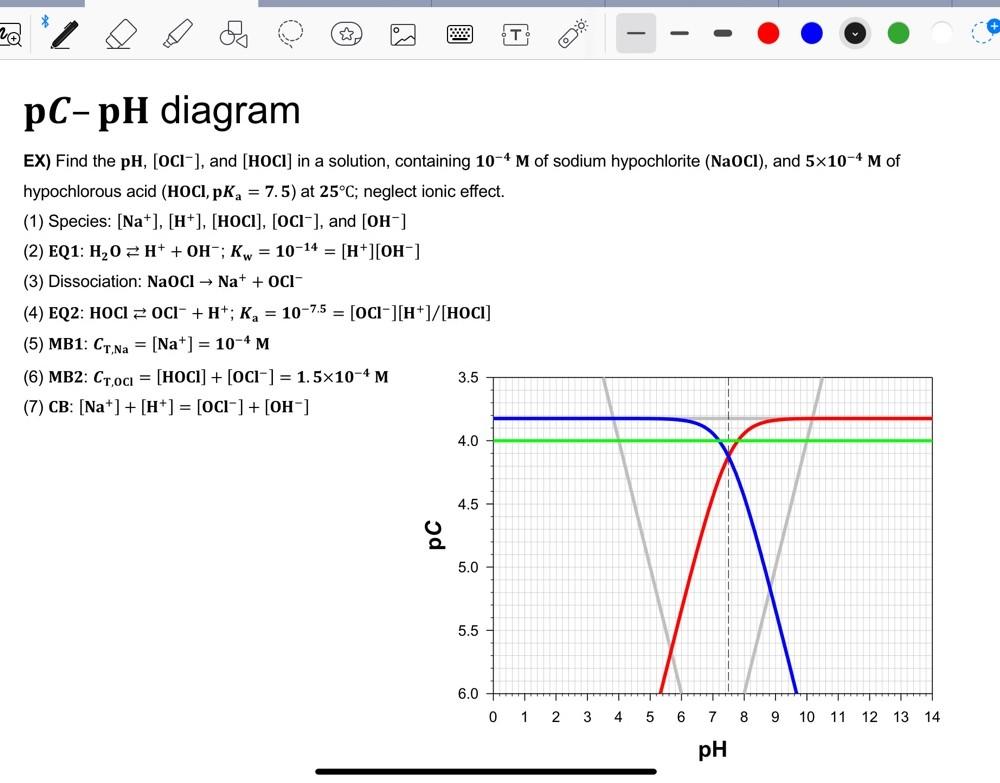
EX) Find the pH,[OCl], and [HOCl] in a solution, containing 104M of sodium hypochlorite ( NaOCl), and 5104M of hypochlorous acid (HOClpKa=7.5) at 25C; neglect ionic effect. (1) Species: [Na+],[H+],[HOCl],[OCl], and [OH] (2) EQ1: H2OH++OH;Kw=1014=[H+][OH] (3) Dissociation: NaOClNa++OCl (4) EQ2: HOClOCl+H+;Ka=107.5=[OCl][H+]/[HOCl] (5) MB1: CT,Na=[Na+]=104M (6) MB2: CT,OCl=[HOCl]+[OCl]=1.5104M (7) CB:[Na+]+[H+]=[OCl]+[OH] EX) Find the pH,[OCl], and [HOCl] in a solution, containing 104M of sodium hypochlorite ( NaOCl), and 5104M of hypochlorous acid (HOClpKa=7.5) at 25C; neglect ionic effect. (1) Species: [Na+],[H+],[HOCl],[OCl], and [OH] (2) EQ1: H2OH++OH;Kw=1014=[H+][OH] (3) Dissociation: NaOClNa++OCl (4) EQ2: HOClOCl+H+;Ka=107.5=[OCl][H+]/[HOCl] (5) MB1: CT,Na=[Na+]=104M (6) MB2: CT,OCl=[HOCl]+[OCl]=1.5104M (7) CB:[Na+]+[H+]=[OCl]+[OH]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


