Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Examination of the Fischer esterification mechanism continues. Overall reaction (ungraded): .. H3C-OH Part 2 of 2: Intermediate species (from Part 1): H-CI: Part 1
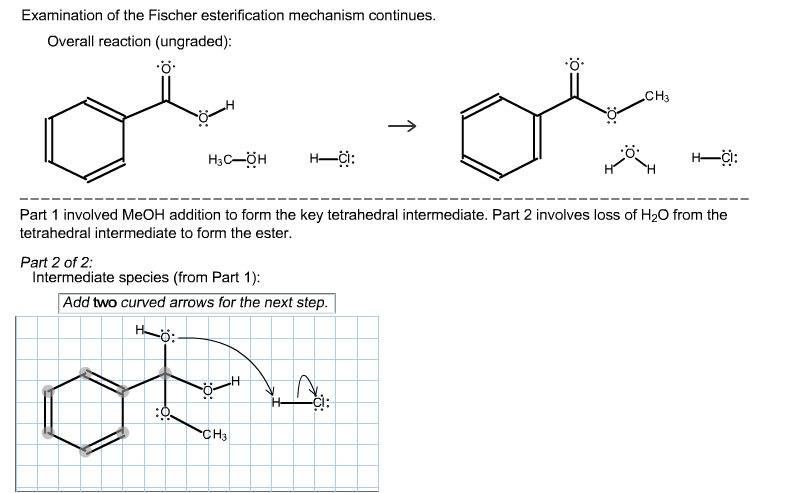
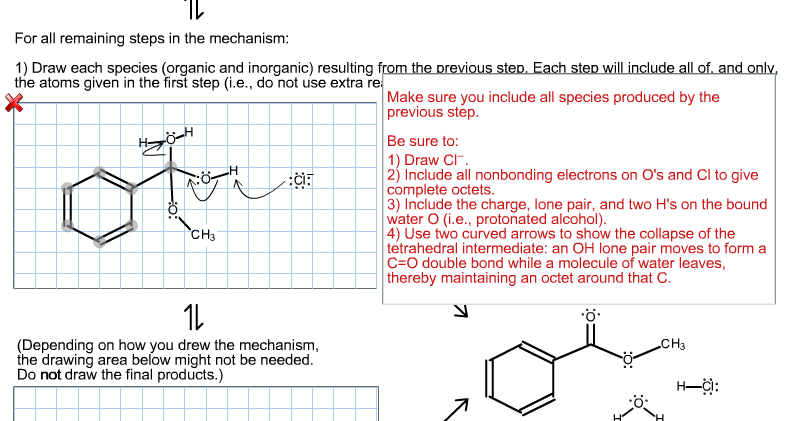
Examination of the Fischer esterification mechanism continues. Overall reaction (ungraded): .. H3C-OH Part 2 of 2: Intermediate species (from Part 1): H-CI: Part 1 involved MeOH addition to form the key tetrahedral intermediate. Part 2 involves loss of HO from the tetrahedral intermediate to form the ester. Add two curved arrows for the next step. CH3 CH3 -CI: H-CI: For all remaining steps in the mechanism: 1) Draw each species (organic and inorganic) resulting from the previous step. Each step will include all of, and only, the atoms given in the first step (i.e., do not use extra re ota CH3 H CIF 1L (Depending on how you drew the mechanism, the drawing area below might not be needed. Do not draw the final products.) Make sure you include all species produced by the previous step. Be sure to: 1) Draw CI. 2) Include all nonbonding electrons on O's and Cl to give complete octets. 3) Include the charge, lone pair, and two H's on the bound water O (i.e., protonated alcohol). 4) Use two curved arrows to show the collapse of the tetrahedral intermediate: an OH lone pair moves to form a C-O double bond while a molecule of water leaves, thereby maintaining an octet around that C. CH3 H-CI:
Step by Step Solution
★★★★★
3.44 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
This is an esterification reaction in which carboxylic acid reacts w...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


