Answered step by step
Verified Expert Solution
Question
1 Approved Answer
EXAMPLE 5.1 The rate at which a metal alloy oxidizes in an oxygen-containing atmosphere is a typical example of the practical utility of the
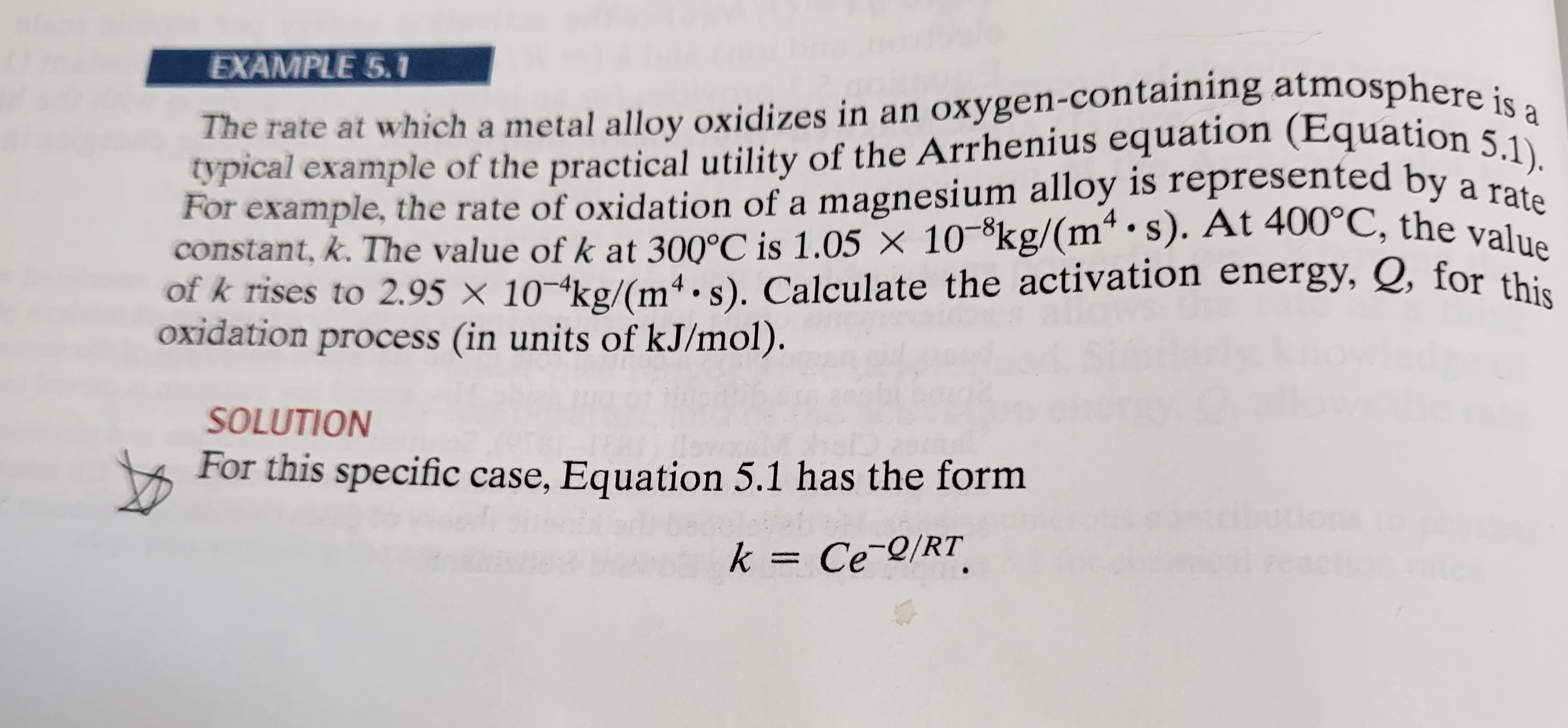
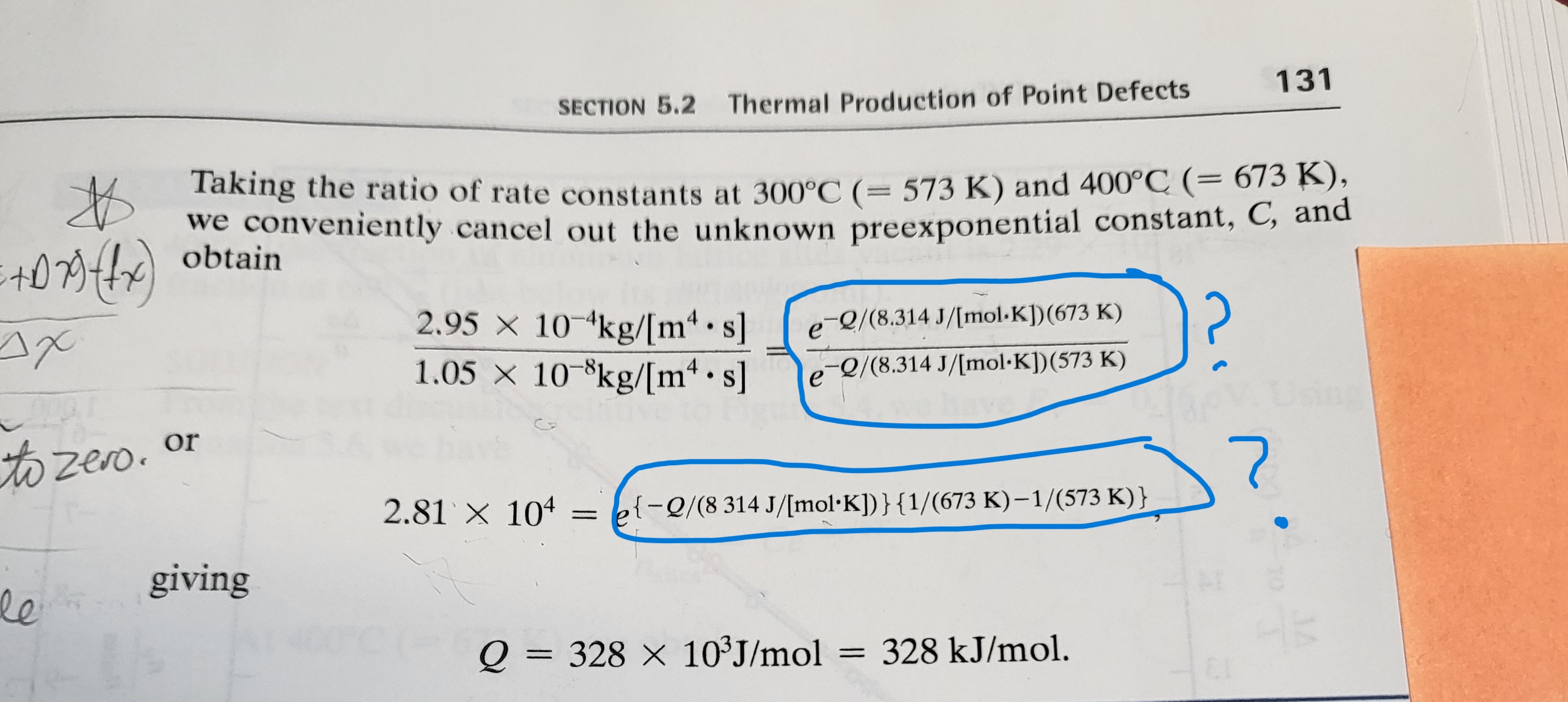
EXAMPLE 5.1 The rate at which a metal alloy oxidizes in an oxygen-containing atmosphere is a typical example of the practical utility of the Arrhenius equation (Equation 5.1). For example, the rate of oxidation of a magnesium alloy is represented by a rate constant, k. The value of k at 300C is 1.05 10kg/(ms). At 400C, the value of k rises to 2.95 10-4kg/(ms). Calculate the activation energy, Q, for this oxidation process (in units of kJ/mol). SOLUTION For this specific case, Equation 5.1 has the form k = CeQ/RT 4 * 5+079) (fx) 2.95 x 10 kg/[m. s] Taking the ratio of rate constants at 300C (= 573 K) and 400C (= 673 K), we conveniently cancel out the unknown preexponential constant, C, and obtain SECTION 5.2 Thermal Production of Point Defects 131 e-Q/(8.314 J/[mol.K]) (673 K) 1.05 x 10 8kg/[m.s] e -2/(8.314 J/[mol.K]) (573 K) to zero. or 2.81 x 10 = {-2/(8 314 J/[molK])} {1/(673 K)1/(573 K) } le giving Q = 328 x 103J/mol = 328 kJ/mol.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


