Question: EXAMPLE 8.2 (Single-stage extraction) One thousand kilograms of an aqueous solution containing 50% acetone is contacted with 800 kg of chlorobenzene containing 0.5 mass% acetone
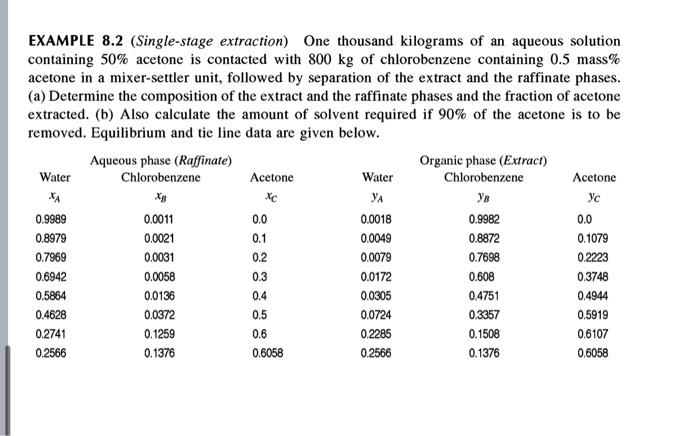
EXAMPLE 8.2 (Single-stage extraction) One thousand kilograms of an aqueous solution containing 50% acetone is contacted with 800 kg of chlorobenzene containing 0.5 mass% acetone in a mixer-settler unit, followed by separation of the extract and the raffinate phases. (a) Determine the composition of the extract and the raffinate phases and the fraction of acetone extracted. (b) Also calculate the amount of solvent required if 90% of the acetone is to be removed. Equilibrium and tie line data are given below. Aqueous phase (Raffinate) Organic phase (Extract) Water Chlorobenzene Acetone Water Chlorobenzene Acetone 24 YA Ys 0.9989 0.0011 0.0 0.0018 0.9982 0.0 0.8979 0.0021 0.0049 0.8872 0.1079 0.7969 0.0031 0.2 0.0079 0.7698 0.2223 0.6942 0.0058 0.0172 0.608 0.3748 0.5864 0.0136 0.4 0.0305 0.4751 0.4944 0.4628 0.0372 0.5 0.0724 0.3357 0.5919 0.2741 0.1259 0.6 0.2285 0.1508 0.6107 0.2566 0.1376 0.6058 0.2566 0.1376 0.6058 XC 0.1 0.3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


