Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Example of a simultaneous diffusion and a heterogeneous reaction process is the diffusion controlled combustion of coal in a fluidized bed to produce energy by
Example of a simultaneous diffusion and a heterogeneous reaction process is the diffusion controlled combustion of coal in a fluidized bed to produce energy by the heat of combustion. Pulverized coal particles are fluidized within a hot combustion chamber, where oxygen in the air reacts with coal to produce carbon monoxide andor carbon dioxide gas.
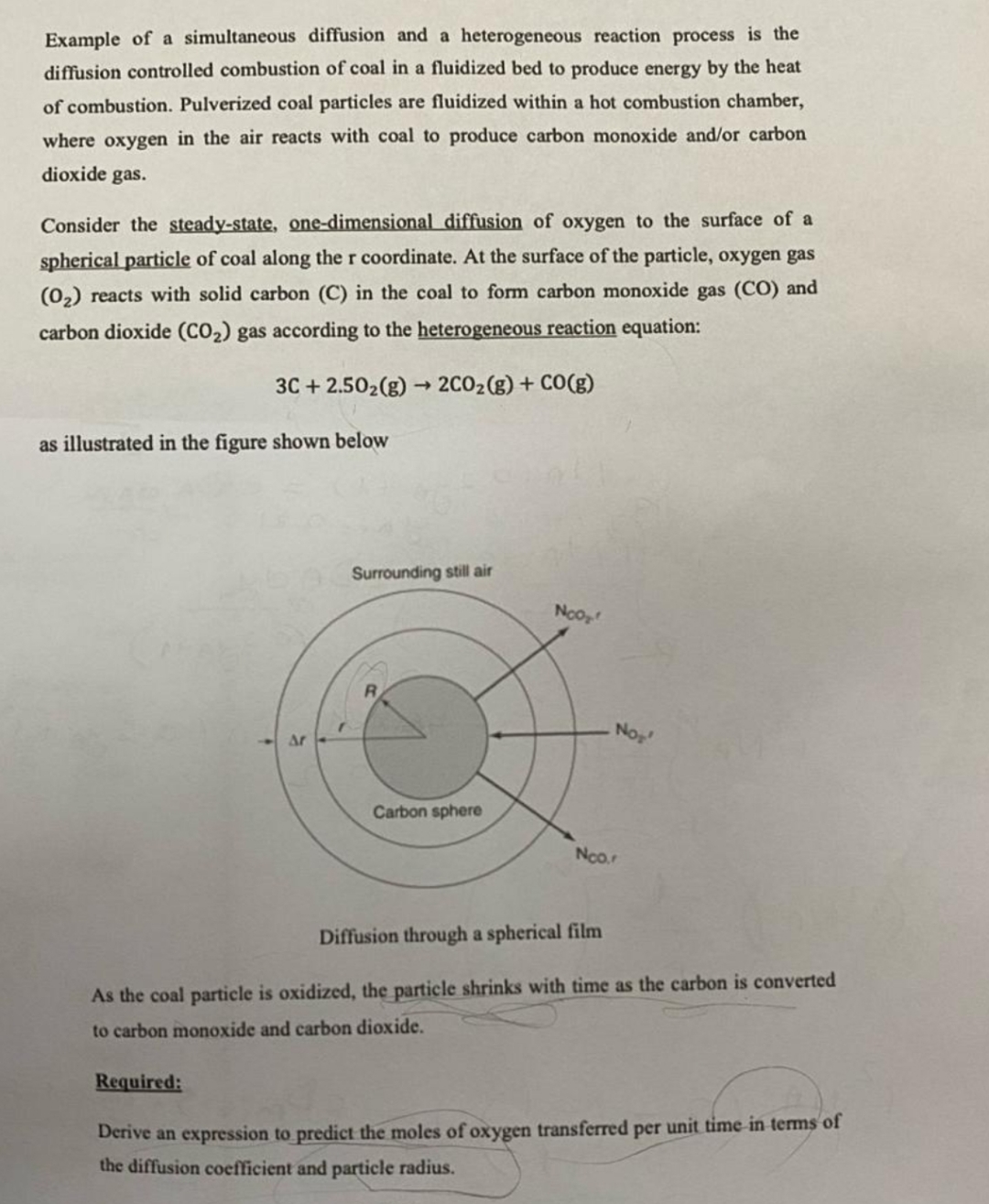
Consider the steadystate, onedimensional diffusion of oxygen to the surface of a spherical particle of coal along the coordinate. At the surface of the particle, oxygen gas reacts with solid carbon in the coal to form carbon monoxide gas and carbon dioxide gas according to the heterogeneous reaction equation:
as illustrated in the figure shown below
Diffusion through a spherical film
As the coal particle is oxidized, the particle shrinks with time as the carbon is converted to carbon monoxide and carbon dioxide.
Required:
Derive an expression to predict the moles of oxygen transferred per unit time in terms of the diffusion coefficient and particle radius
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


