Answered step by step
Verified Expert Solution
Question
1 Approved Answer
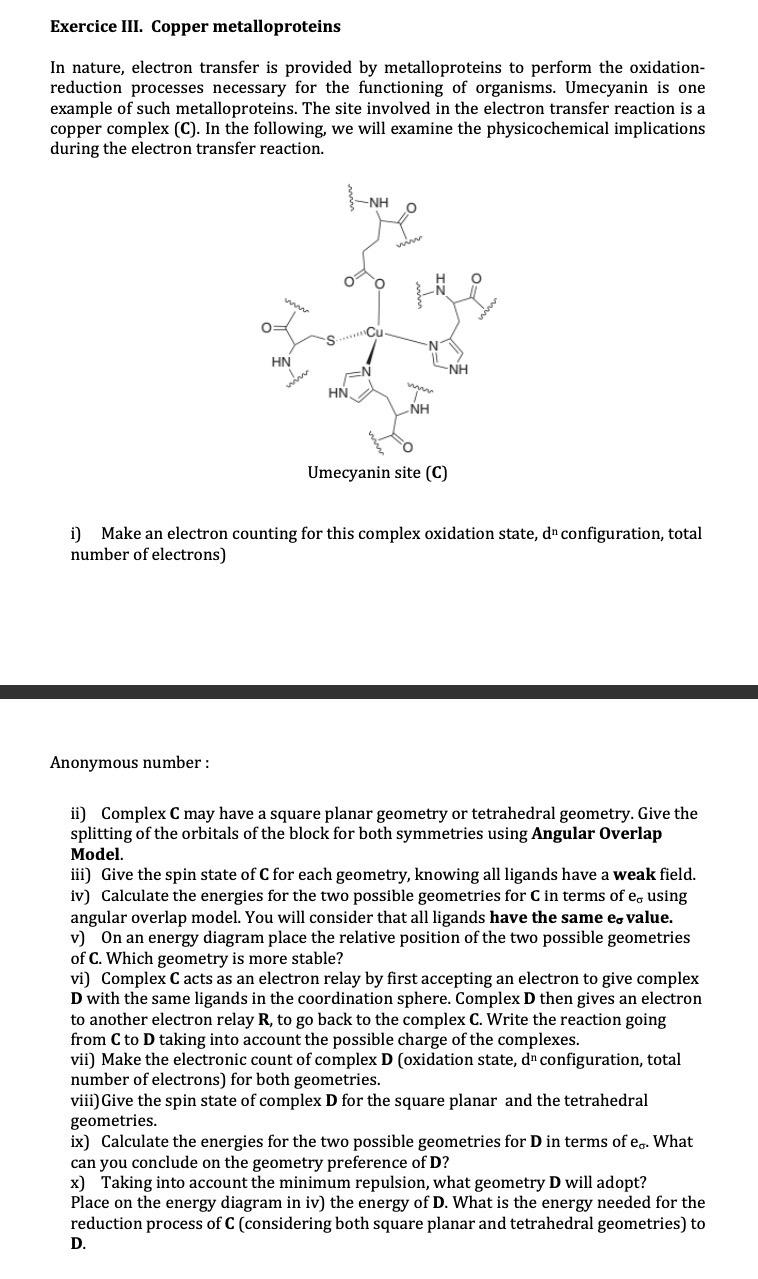
Exercice III. Copper metalloproteins In nature, electron transfer is provided by metalloproteins to perform the oxidationreduction processes necessary for the functioning of organisms. Umecyanin is
Exercice III. Copper metalloproteins
In nature, electron transfer is provided by metalloproteins to perform the oxidationreduction processes necessary for the functioning of organisms. Umecyanin is one example of such metalloproteins. The site involved in the electron transfer reaction is a copper complex C In the following, we will examine the physicochemical implications during the electron transfer reaction.
i Make an electron counting for this complex oxidation state, configuration, total number of electrons
Anonymous number :
ii Complex C may have a square planar geometry or tetrahedral geometry. Give the splitting of the orbitals of the block for both symmetries using Angular Overlap Model.
iii Give the spin state of for each geometry, knowing all ligands have a weak field.
iv Calculate the energies for the two possible geometries for in terms of using angular overlap model. You will consider that all ligands have the same value.
v On an energy diagram place the relative position of the two possible geometries of Which geometry is more stable?
vi Complex C acts as an electron relay by first accepting an electron to give complex D with the same ligands in the coordination sphere. Complex then gives an electron to another electron relay to go back to the complex Write the reaction going from to taking into account the possible charge of the complexes.
vii Make the electronic count of complex oxidation state, configuration, total number of electrons for both geometries.
viiiGive the spin state of complex for the square planar and the tetrahedral geometries.
ix Calculate the energies for the two possible geometries for in terms of What can you conclude on the geometry preference of
Taking into account the minimum repulsion, what geometry will adopt?
Place on the energy diagram in iv the energy of What is the energy needed for the reduction process of considering both square planar and tetrahedral geometries to D
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


