Answered step by step
Verified Expert Solution
Question
1 Approved Answer
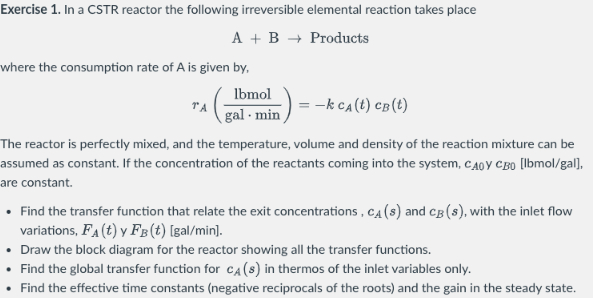
Exercise 1 . In a CSTR reactor the following irreversible elemental reaction takes place A + B Products where the consumption rate of A is
Exercise In a CSTR reactor the following irreversible elemental reaction takes place
Products
where the consumption rate of is given by
The reactor is perfectly mixed, and the temperature, volume and density of the reaction mixture can be
assumed as constant. If the concentration of the reactants coming into the system, y
are constant.
Find the transfer function that relate the exit concentrations, and with the inlet flow
variations, y
Draw the block diagram for the reactor showing all the transfer functions.
Find the global transfer function for in thermos of the inlet variables only.
Find the effective time constants negative reciprocals of the roots and the gain in the steady state.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


