Question
Exercise 11. Consider a hydrogen-oxygen fuel cell. a) What volume of H2(g), stored at 25C at a pressure of 155 atm, would be needed
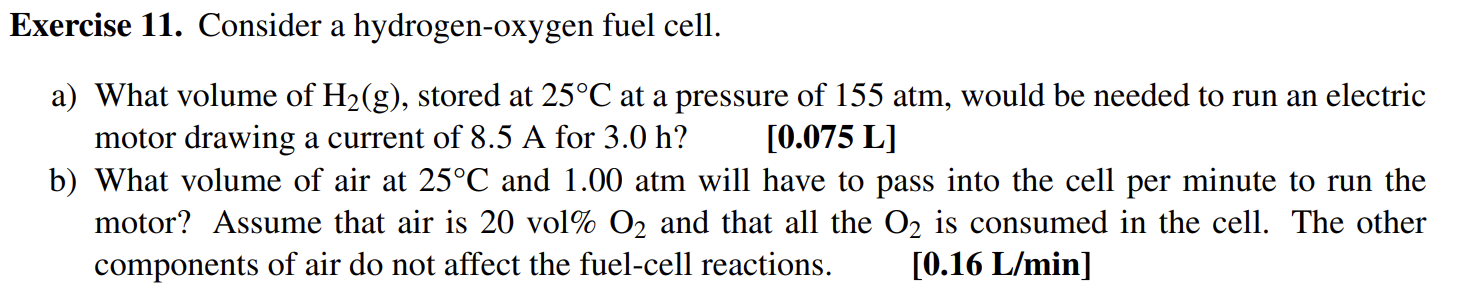
Exercise 11. Consider a hydrogen-oxygen fuel cell. a) What volume of H2(g), stored at 25C at a pressure of 155 atm, would be needed to run an electric motor drawing a current of 8.5 A for 3.0 h? [0.075 L] b) What volume of air at 25C and 1.00 atm will have to pass into the cell per minute to run the motor? Assume that air is 20 vol% O2 and that all the O2 is consumed in the cell. The other components of air do not affect the fuel-cell reactions. [0.16 L/min]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Physics Reasoning and Relationships
Authors: Nicholas Giordano
2nd edition
840058195, 9781285225340 , 978-0840058195
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



