Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Exercise 3 . In an adiabatic chemical reactor the following reaction takes place A + B C where the reaction kinetics is of order cero
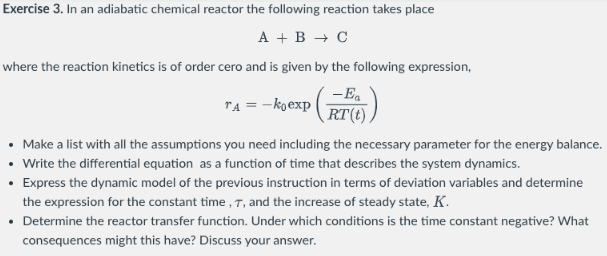
Exercise In an adiabatic chemical reactor the following reaction takes place
where the reaction kinetics is of order cero and is given by the following expression,
exp
Make a list with all the assumptions you need including the necessary parameter for the energy balance.
Write the differential equation as a function of time that describes the system dynamics.
Express the dynamic model of the previous instruction in terms of deviation variables and determine
the expression for the constant time, and the increase of steady state,
Determine the reactor transfer function. Under which conditions is the time constant negative? What
consequences might this have? Discuss your answer.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


