Answered step by step
Verified Expert Solution
Question
1 Approved Answer
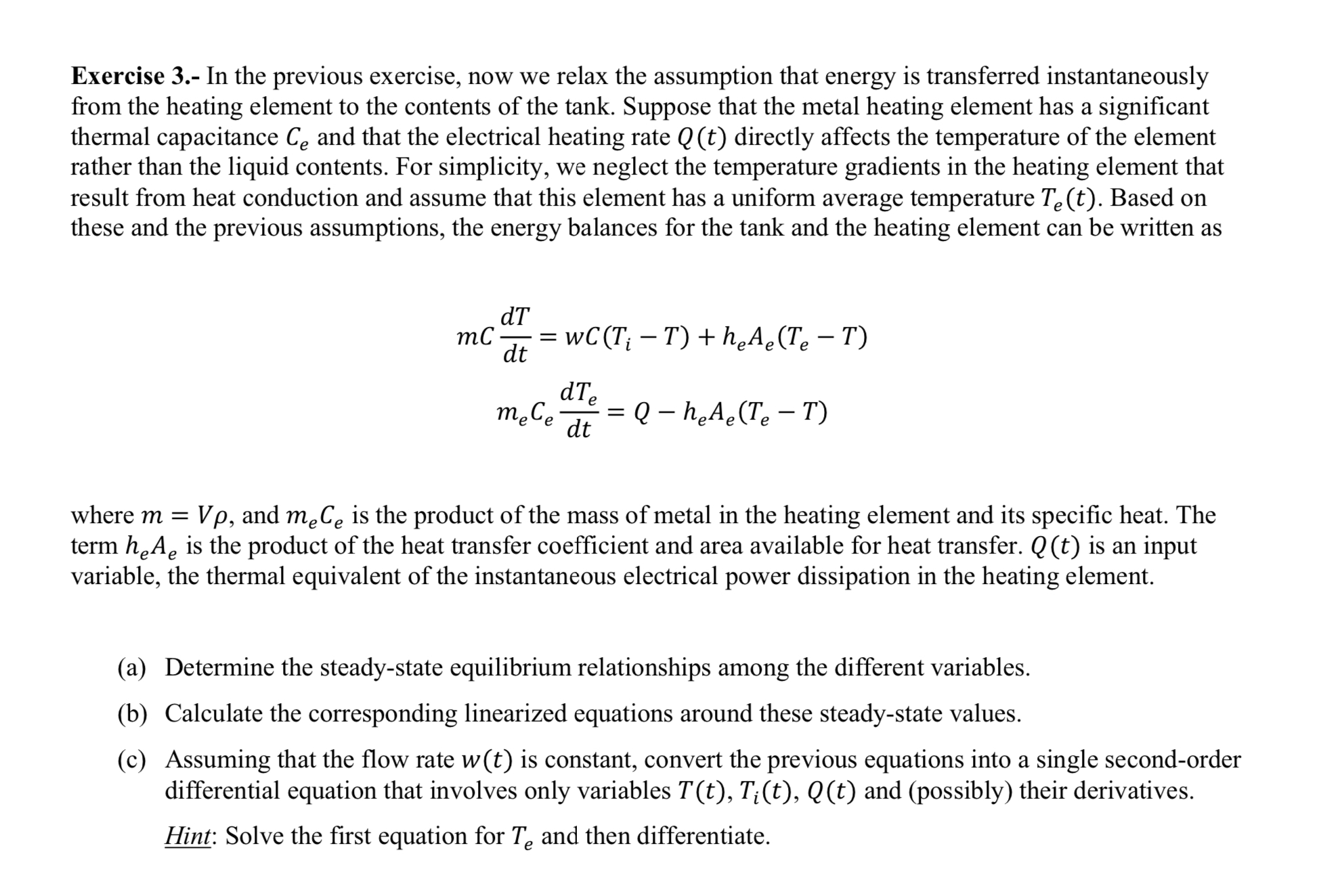
Exercise 3 . - In the previous exercise, now we relax the assumption that energy is transferred instantaneously from the heating element to the contents
Exercise In the previous exercise, now we relax the assumption that energy is transferred instantaneously
from the heating element to the contents of the tank. Suppose that the metal heating element has a significant
thermal capacitance and that the electrical heating rate directly affects the temperature of the element
rather than the liquid contents. For simplicity, we neglect the temperature gradients in the heating element that
result from heat conduction and assume that this element has a uniform average temperature Based on
these and the previous assumptions, the energy balances for the tank and the heating element can be written as
where and is the product of the mass of metal in the heating element and its specific heat. The
term is the product of the heat transfer coefficient and area available for heat transfer. is an input
variable, the thermal equivalent of the instantaneous electrical power dissipation in the heating element.
a Determine the steadystate equilibrium relationships among the different variables.
b Calculate the corresponding linearized equations around these steadystate values.
c Assuming that the flow rate is constant, convert the previous equations into a single secondorder
differential equation that involves only variables and possibly their derivatives.
Hint: Solve the first equation for and then differentiate.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


