Answered step by step
Verified Expert Solution
Question
1 Approved Answer
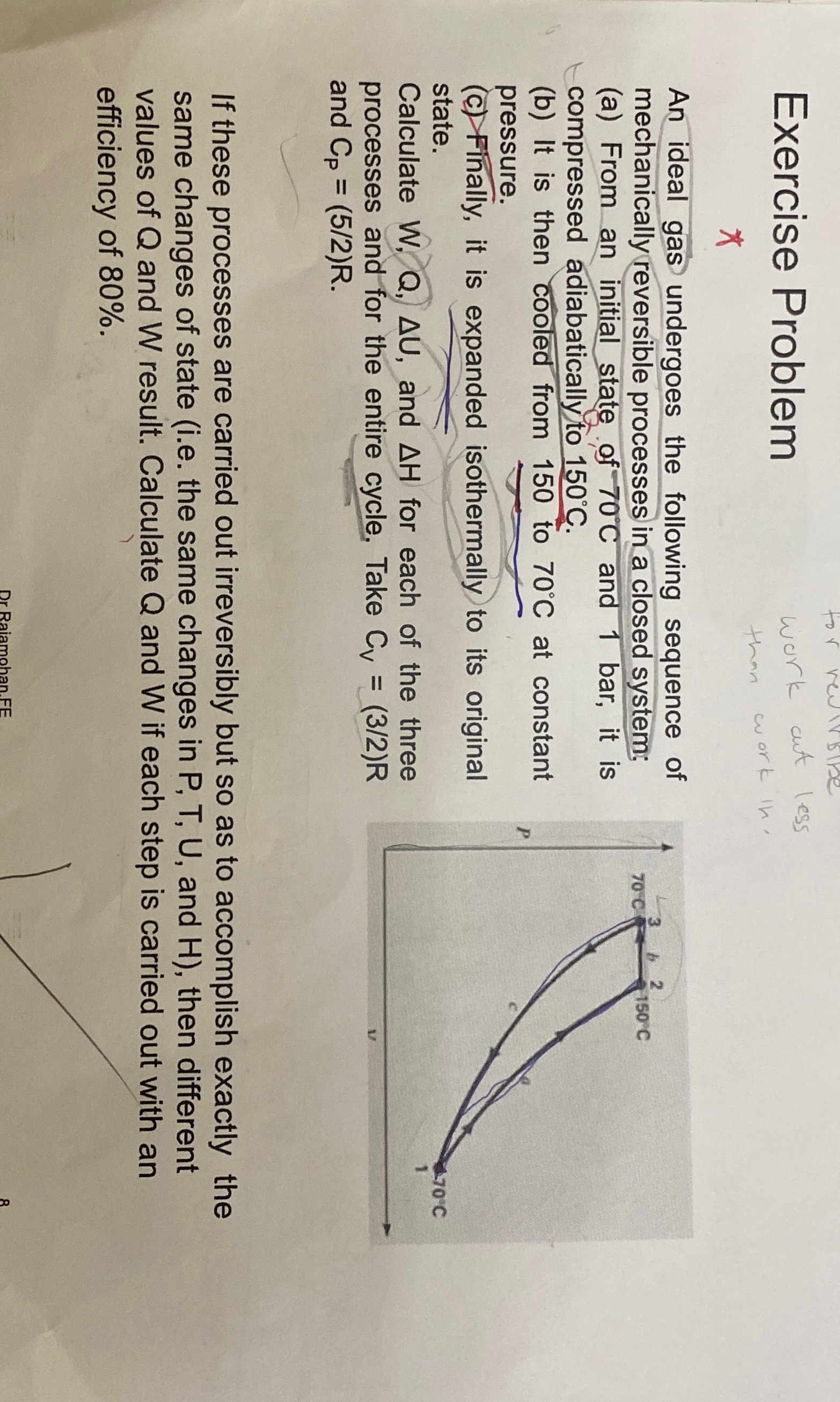
Exercise Problem * An ideal gas undergoes the following sequence of mechanically reversible processes in a closed system: ( a ) From an initial state
Exercise Problem
An ideal gas undergoes the following sequence of mechanically reversible processes in a closed system:
a From an initial state of and bar, it is compressed adiabatically to
b It is then cooled from to at constant pressure.
c Finally, it is expanded isothermally to its original state.
Calculate and for each of the three processes and for the entire cycle. Take and
If these processes are carried out irreversibly but so as to accomplish exactly the same changes of state ie the same changes in and then different values of and result. Calculate and if each step is carried out with an efficiency of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


