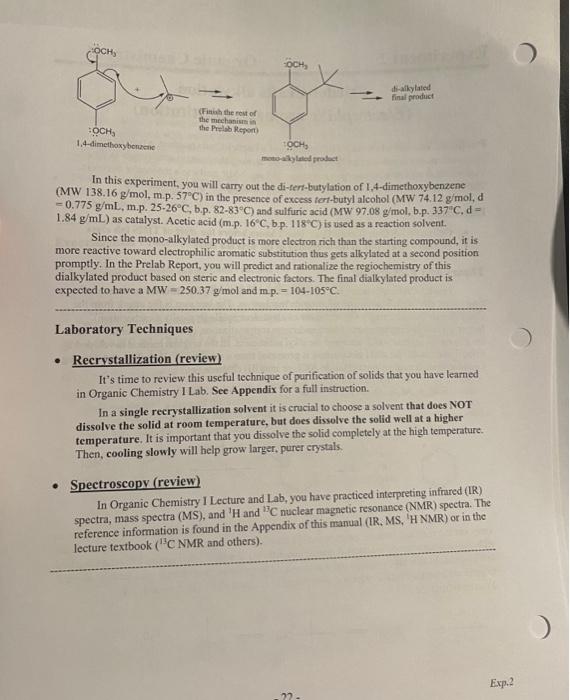
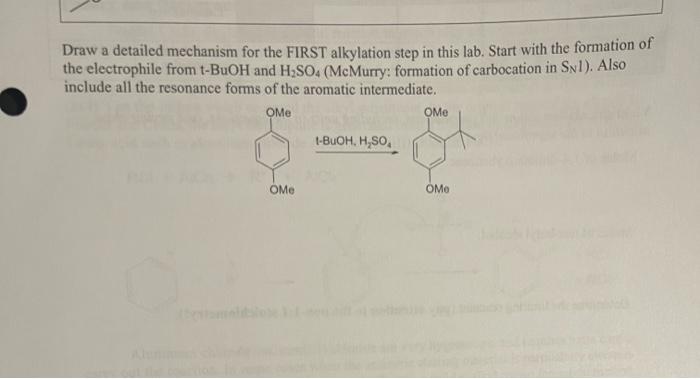
Experiment 2 Organic Chemistry II Friedel-Crafts alkylation (Electrophilic aromatic substitution) Purpose: You will carry out a milder version of a Friedel-Crafts reaction, a type of an electronic aromatic substitution. You will practice drawing the reaction mechanism in detail, You will also review spectroscopy, and assign MS and NMR peaks to a molecular structure. These practices will further polish your skills to be successful in organic chemistry Due dates Online Prelab Quiz (Course Den): Due 2 hours before the start of THIS lab class Postlab Report: Due at the beginning of the next lab class . Theory and Background Friedel-Crafts alkylation is a useful reaction to attach alky substituents to an aromatic ring via an electrophilic aromatic substitution mechanism. The key step is the attack from the electron-rich aromatic ring's electrons to an alkyl electrophile (typically a carbocation), forming a cationic intermediate, which undergoes rearomatization upon removal of the H atom (as H) on the carbon which accepted the alkyl substituent. In the original method developed by Friedel and Crafts, the alkyl electrophile is generated from a reaction of alkyhalide and a strong Lewis acid such as aluminum chloride AICI, or iron(III) chloride Fecho: RCI + AICI R' + AICL + HCI + AICI Aluminum chloride and iron(III) chloride are very hygroscopic and requires extra care to carry out the reaction. In some cases when the aromatic starting material is particularly clectron- rich (e.g. having a strong electron-donating substituent), a milder alkyl cation formation method can be used. In this lab, you will use a strong acid to protonate and dchydrate a tertiary alcohol to generate a tertiary cation, which is subsequently is attacked by electron-rich 1.4- dimethoxybenzene: HO OH . Hysok HSO E2 -21- Exp.2 OCH, skylated final product HOCHS 1,4-dimethoxybenzen Finish the rest of the mechanism the Pelab Report OCH motylated product In this experiment, you will carry out the di-tert-butylation of 1,4-dimethoxybenzene (MW 138.16 g/mol, m.p. 57C) in the presence of excess tert-butyl alcohol (MW 74.12 g mold = 0.775 g/mL, m.p. 25-26C, b.p. 82-83C) and sulfuric acid (MW 97.08 g/mol, b.p.337", d- 1.84 g/mL) as catalyst. Acetic acid (m.p. 16C, b.p. 118C) is used as a reaction solvent. Since the mono-alkylated product is more electron rich than the starting compound, it is more reactive toward electrophilic aromatic substitution thus gets alkylated at a second position promptly. In the Prelab Report, you will predict and rationalize the regiochemistry of this dialkylated product based on steric and electronic factors. The final dialkylated product is expected to have a MW=250.37 g/mol and mp. = 104-105C. Laboratory Techniques Recrystallization (review) It's time to review this useful technique of purification of solids that you have learned in Organic Chemistry I Lab. See Appendix for a full instruction In a single recrystallization solvent it is crucial to choose a solvent that does NOT dissolve the solid at room temperature, but does dissolve the solid well at a higher temperature. It is important that you dissolve the solid completely at the high temperature. Then, cooling slowly will help grow larger, purer crystals. Spectroscopy (review) In Organic Chemistry I Lecture and Lab, you have practiced interpreting infrared (IR) spectra, mass spectra (MS), and 'H and C nuclear magnetic resonance (NMR) spectra. The reference information is found in the Appendix of this manual (IR, MS, 'H NMR) or in the lecture textbook ("C NMR and others) Exp.2 . Safety Notes Sulfuric acid is a strong acid and a potent oxidizer. Wear gloves. In case of skin contact, wipe off the liquid with paper towel first and then wash with copious amount of water. DO NOT wash with water before wiping off the bulk sulfuric acid: the dilution of sulfuric acid with water is highly exothermic and you would burn from heat. Dilution of sulfuric acid and acetic acid with water is both very exothermic. Precool the acid-containing solution, and add water dropwise, very carefully, to avoid dangerous boiling and bumping of the acidic liquid. . Experimental Procedure Electrophilie aromatic substitution reaction (1) In a reaction tube, measure 0.120 g of 1,4-dimethoxybenzene. (2) Add 0.4 mL of acetic acid via a syringe and dissolve the solid with gentle heating in a sand bath on low setting (do not overheat). . It may be necessary (3) Add 0.200 mL (200 L) of tert-butyl alcohol via a micropipetter alcohol as tes melting to warm and me she (review Exp. 1 Techniques). points around room (4) Cool the mixture in an ice bath Temperature (5) Measure 0.4 mL of sulfuric acid in a clean and dry plastic syringe. There are all Add carefully a drop of the sulfuric acid to the pre-cooled reaction precautions for the tube and mix thoroughly. Repeat the dropwise addition and mixing highly exothermic after each drop for all 0.4 mL sulfuric acid. reaction upon addition of H.SO At the end of this addition, solid reaction product should have separated. Stir the mixture thoroughly with a glass rod, remove the reaction tube from the ice bath, and allow it to warm to room temperature and remain at 20-25C for at least 10 minutes to complete the reaction "Rinse the syringe with copious amount of cold water, rinse with acetone, and air-dry for the next use. Removal of water-soluble acids Dllution of both (6) Cool the mixture in ice again. In a separate test tube, have 2.5 mL H:SO, and acetic acid of water ready. Very carefully add one drop of the water to the reaction in water is exothermic mixture, stir thoroughly with the glass rod, and continue to add the so caution is necessary remainder of the water dropwise with cooling and stirring (7) Remove the diluted aqueous solution from the organic solid product This dilution with by vacuum filtration (Hirsch funnel, side-arm flask, vacuum hose). water allows to separate these acidi from the organic reaction product Exp.2 - 23 - Exp.2 O Recrystallization (8) Place the solid (crude product) in a clean and dry test tube. Add 0.5-1 mL of methanol and heat in a warm water bath. Without boiling off the solvent, dissolve all the crude solid near the boiling temperature. Allow the hot solution to cool to room temperature and then to 0C in ice water bath. (While waiting, clean and dry the vacuum filtration apparatus and prepare ice-cold methanol.) (9) Collect the crystals by vacuum filtration. Rinse the crystals by minimum amount of cold methanol. Dry the crystals by passing air over the Hirsch funnel. Obtain the mass of this purified product and the melting point Clean-up All the liquid waste should be collected in the designated liquid waste container. The solid product, once the m.p. and the mass is measured, is collected in a solid waste container. Draw a detailed mechanism for the FIRST alkylation step in this lab. Start with the formation of the electrophile from t-BuOH and H2SO4 (McMurry: formation of carbocation in Syl). Also include all the resonance forms of the aromatic intermediate. OME Me t-BUOH, H,SO OMe OMo