Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Experimental data mass (g) and volume (mL) of a substance was gathering, recorded, and plotted in Figure 1. a. Correctly label the graph axis
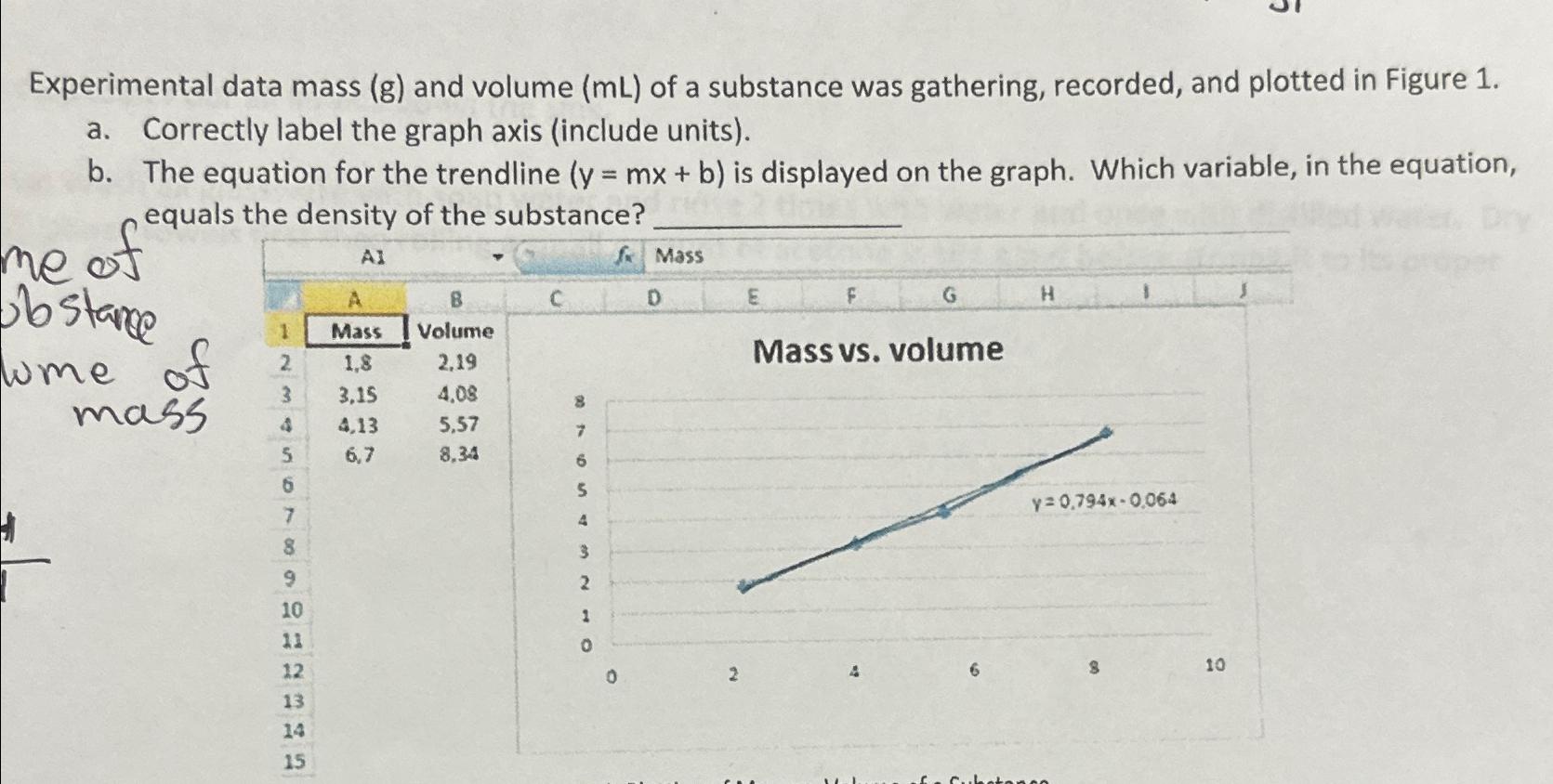
Experimental data mass (g) and volume (mL) of a substance was gathering, recorded, and plotted in Figure 1. a. Correctly label the graph axis (include units). b. The equation for the trendline (y = mx + b) is displayed on the graph. Which variable, in the equation, equals the density of the substance? AI me of obstance lume of mass 1 2 3 5 6 7 8 9 10 11 12 13 14 15 A Mass 1,8 3,15 4,13 6,7 B Volume 2,19 4,08 5,57 8.34 7 6 5 4 3 2 1 0 f Mass 0 D 2 E F G Mass vs. volume H y=0,794x-0,064 10
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The experimental data shown in your image includes values for mass in grams and volume in milliliters of a substance The data is plotted on a graph which is labeled Mass vs volume and represents mass on the yaxis and volume on the xaxis However typically when creating a mass vs volume graph mass should be plotted on the xaxis independent variable and volume on the yaxis dependent variable but convention can vary The correct labeling for the axes based on the data in the spreadsheet and corresponding to the trendline equation provided would be a For the xaxis Volume mL and for the yaxis Mass g based on the spreadsheet data and the graph title b The graph includes a trendline with the equation y 0794x 0064 In the context of this experiment y represents the mass in grams x represents the volume in milliliters m is the slope of the line and b is the yintercept The slope m 0794 of the trendline represents the ratio of mass to volume which is the density of the substance in grams per milliliter ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


