Answered step by step
Verified Expert Solution
Question
1 Approved Answer
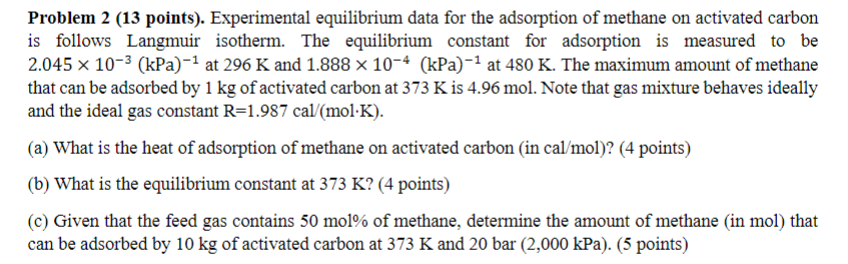
Experimental equilibrium data for the adsorption of methane on activated carbon is follows Langmuir isotherm. The equilibrium constant for adsorption is measured to be 2
Experimental equilibrium data for the adsorption of methane on activated carbon
is follows Langmuir isotherm. The equilibrium constant for adsorption is measured to be
at and at The maximum amount of methane
that can be adsorbed by of activated carbon at is mol. Note that gas mixture behaves ideally
and the ideal gas constant cal
a What is the heat of adsorption of methane on activated carbon in calmol
b What is the equilibrium constant at
c Given that the feed gas contains mol of methane, determine the amount of methane in mol that
can be adsorbed by of activated carbon at and bar kPa
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


