Answered step by step
Verified Expert Solution
Question
1 Approved Answer
explain and show work for question 10 The standard free energy change for different reactions are given below. Use this chart to answer questions 8
explain and show work for question 10 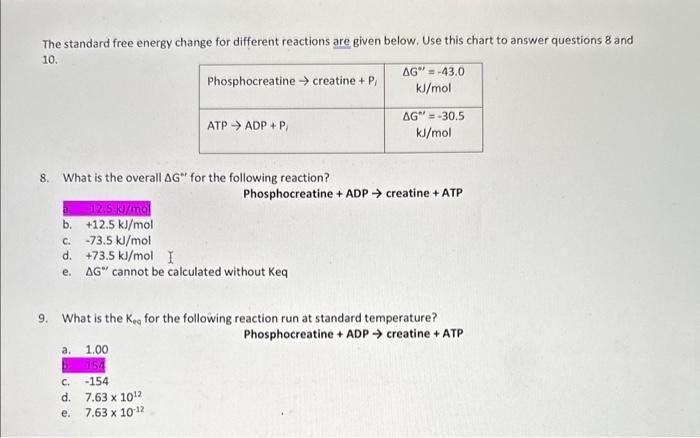
The standard free energy change for different reactions are given below. Use this chart to answer questions 8 and 10. 8. What is the overall G for the following reaction? 2. 12.5kj/mol Phosphocreatine + ADP creatine + ATP b. +12.5kJ/mol c. 73.5kJ/mol d. +73.5kJ/mol e. G cannot be calculated without Keq 9. What is the Keq for the following reaction run at standard temperature? a. 1.00 Phosphocreatine + ADP creatine + ATP b. 154 c. 154 d. 7.631012 e. 7.631012 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


