Answered step by step
Verified Expert Solution
Question
1 Approved Answer
explain each step in detail please. A distillation column is being designed to produce a distillate product at the rate of 45.1kmoles/hr with the anticipated
explain each step in detail please. 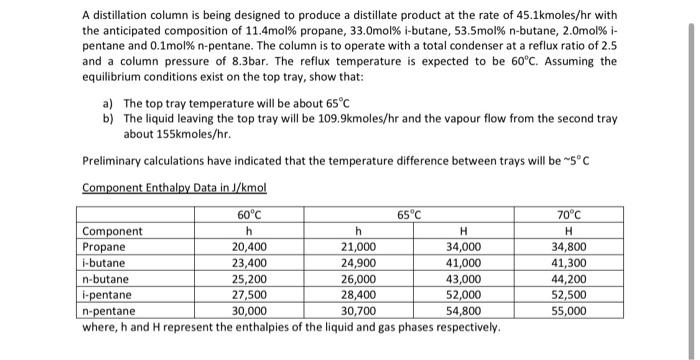
A distillation column is being designed to produce a distillate product at the rate of 45.1kmoles/hr with the anticipated composition of 11.4mol% propane, 33.Omol% i-butane, 53.5mol% n-butane, 2.omol% i- pentane and 0.1mol% n-pentane. The column is to operate with a total condenser at a reflux ratio of 2.5 and a column pressure of 8.3bar. The reflux temperature is expected to be 60C. Assuming the equilibrium conditions exist on the top tray, show that: a) The top tray temperature will be about 65C b) The liquid leaving the top tray will be 109.9kmoles/hr and the vapour flow from the second tray about 155kmoles/hr. Preliminary calculations have indicated that the temperature difference between trays will be 5C Component Enthalpy Data in J/mol 60C 65C 70C Component h h H Propane 20,400 21,000 34,000 34,800 i-butane 23,400 24,900 41,000 41,300 n-butane 25,200 26,000 43,000 44,200 L-pentane 27,500 28,400 52,000 52,500 n-pentane 30,000 30,700 54,800 55,000 where, h and H represent the enthalpies of the liquid and gas phases respectively. H 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


