Answered step by step
Verified Expert Solution
Question
1 Approved Answer
explain how 3A was solved and what it means In Class Exercise 3. Creatinine Kinase catalyzes the following reaction: ATP + Creatine ADP + Phosphocreatine
explain how 3A was solved and what it means 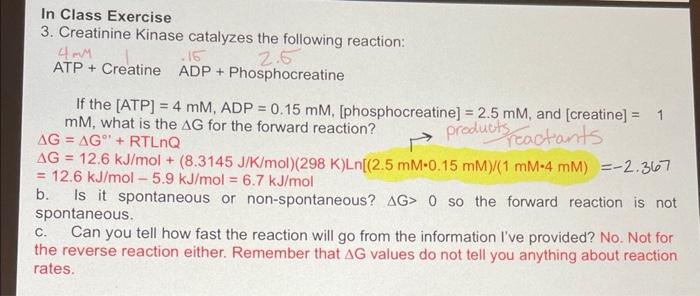
In Class Exercise 3. Creatinine Kinase catalyzes the following reaction: ATP + Creatine ADP + Phosphocreatine If the [ATP]=4mM,ADP=0.15mM, [phosphocreatine ]=2.5mM, and [creatine]=1 mM, what is the G for the forward reaction? G=G+RTLnQ G=12.6kJ/mol+(8.3145J/K/mol)(298K)Ln[(2.5mM0.15mM)/(1mM4mM)=2.367 =12.6kJ/mol5.9kJ/mol=6.7kJ/mol b. Is it spontaneous or non-spontaneous? G>0 so the forward reaction is not spontaneous. c. Can you tell how fast the reaction will go from the information I've provided? No. Not for the reverse reaction either. Remember that G values do not tell you anything about reaction rates 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


