Answered step by step
Verified Expert Solution
Question
1 Approved Answer
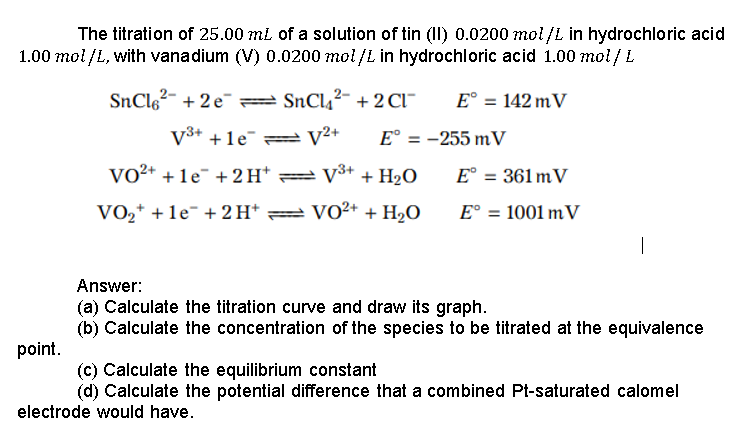
explained step by step please The titration of 25.00 mL of a solution of tin (II) 0.0200 mol/L in hydrochloric acid 1.00 mol/L, with vanadium
explained step by step please
The titration of 25.00 mL of a solution of tin (II) 0.0200 mol/L in hydrochloric acid 1.00 mol/L, with vanadium (V) 0.0200 mol/L in hydrochloric acid 1.00 mol/L SnC162- +2e=SnCl2- + 2 C1" E = 142 mV V3+ +1e = V2+ E = -255 m V VO2+ + 1e" + 2H=V3+ + H2O E = 361 m V VO2+ +1e- + 2H+ = VO2+ + H2O E = 1001 mV | Answer: (a) Calculate the titration curve and draw its graph. (b) Calculate the concentration of the species to be titrated at the equivalence point. (c) Calculate the equilibrium constant (d) Calculate the potential difference that a combined Pt-saturated calomel electrode would haveStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


